JSBB: Volume 2, Issue 1, April 2023 - STRUCTURE & FUNCTION
Concept article.
How does the antidepressant ESCitalopram work & Prospects for Rationally Designing of new 'Antidepressants' .. the 3D-structural perspective.
RACHEDI Abdelkrim
Laboratory of Biotoxicology, Pharmacognosy and biological valorisation of plants, Faculty of Sciences, Department of Biology, University of Saida - Dr Moulay Tahar, 20100 Saida, Algeria.
📧 E. mail: abdelkrim.rachedi@univ-saida.dz
Published: 11 April 2023
Depression is a major public health issue, affecting over 300 million people worldwide. While antidepressant medications have been available for decades, their efficacy and tolerability are variable, and many patients require multiple trials of different medications before finding one that works for them. Furthermore, many of the currently available antidepressants, including escitalopram, have significant side effects.
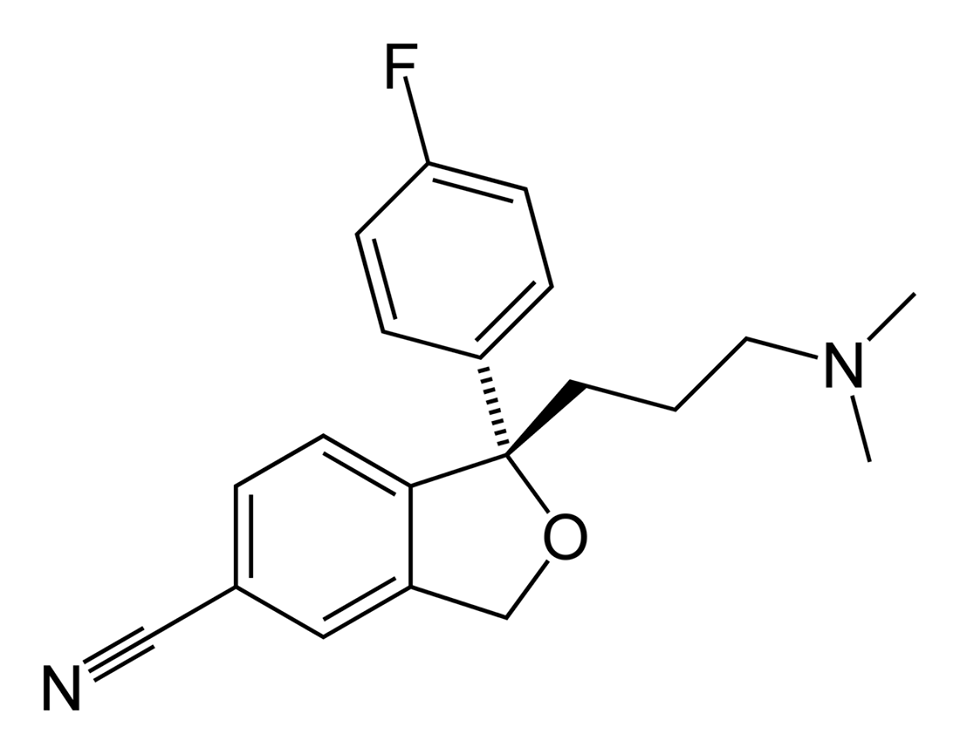
Escitalopram, Figuer 1, is a selective serotonin reuptake inhibitor that increases the availability of serotonin in the brain, leading to increased activation of serotonin receptors and a reduction in symptoms of depression and anxiety. However, it is not effective for all patients, and its side effects can be significant. Therefore, there is a need for the development of new and better antidepressant medications.
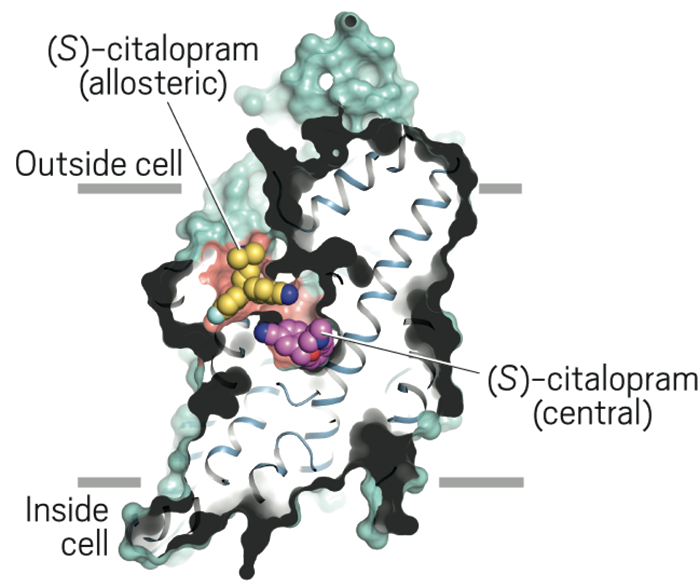
Figure 1. (S)-citalopram binds to both the central (green) and allosteric (blue) binding sites. (Nature)
see: https://cen.acs.org/articles/94/i15/last-scientists-solve-structure-protein.html
see: https://cen.acs.org/articles/94/i15/last-scientists-solve-structure-protein.html
A number of 3D-structures have been determined, using x-ray crystallography, 2016, of the Human Serotonin Transporter (SERT) many in complex forms bound with selected derivatives of the antidepressant ESCitalopram, Figuer 2. The structures contribute towards gaining incites into the working mechanism of the antidepressant and analysing the intriguing discovery that ESCitalopram can also bind to the allosteric site of the Serotonin transporter in addition to the central active site (S1) !!
Follow the SSFS tool links provided below and see the attached images.
Such discovery has set this protein as important target for the new and more effective antidepressants via the Rational Drug Design and alike techniques.
For more details, follow the link:
For more details, follow the link:
The technical papers is available in Nature:
The paper describes the determination of the three-dimensional structure of the SERT, which is a protein that plays a crucial role in the regulation of serotonin levels in the brain. The researchers used X-ray crystallography to determine the structure of the protein, which revealed important insights into how it functions at the molecular level.
The study also explored the binding of different compounds to the serotonin transporter, including antidepressants and amphetamines, which are known to interact with the protein. The researchers found that these compounds bind to specific regions of the protein and alter its function, which can have therapeutic effects in the treatment of depression and other psychiatric disorders.
The paper also describes the development of a computational model that can predict the binding of different compounds to the serotonin transporter. This model could be used to design new drugs that target the protein and improve its function.
Exploration of the 3D-structures and binding detail is available via the SSFS tool, on the Bioinformaticstools.org web-site, University of Saida:
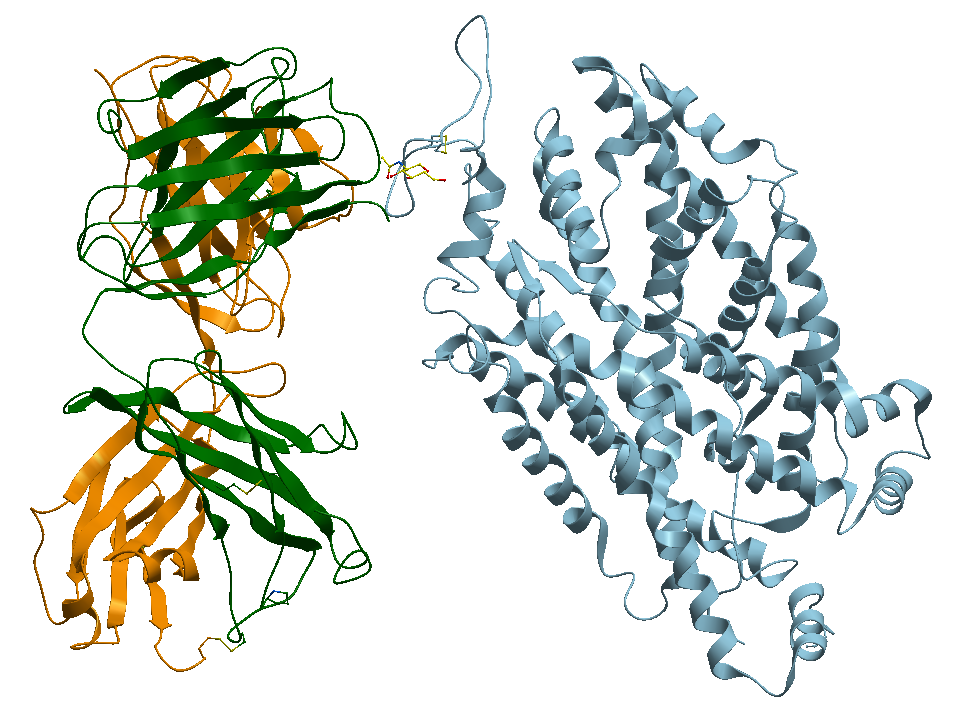
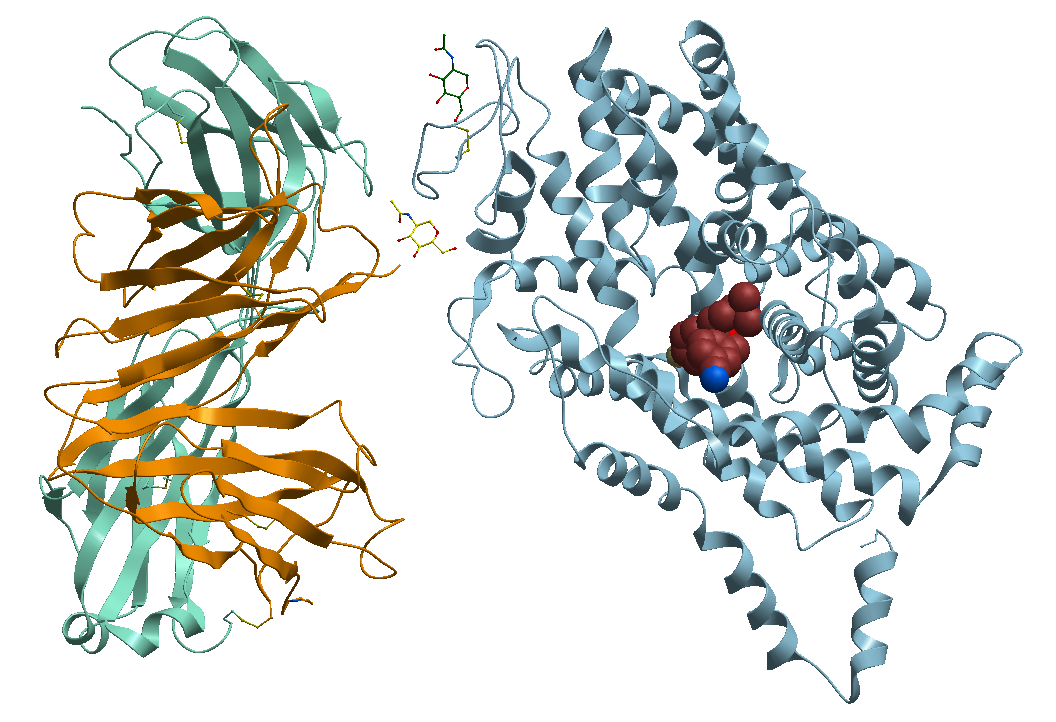
1- The TS2 Human Serotonin Transporter, the non-bound apo form, Figure 3.
Figure 3. X-ray structure of the ts2 human serotonin transporter
https://bioinformaticstools.org/ssfs/ssfs.php?qry=5i6z
https://bioinformaticstools.org/ssfs/ssfs.php?qry=5i6z
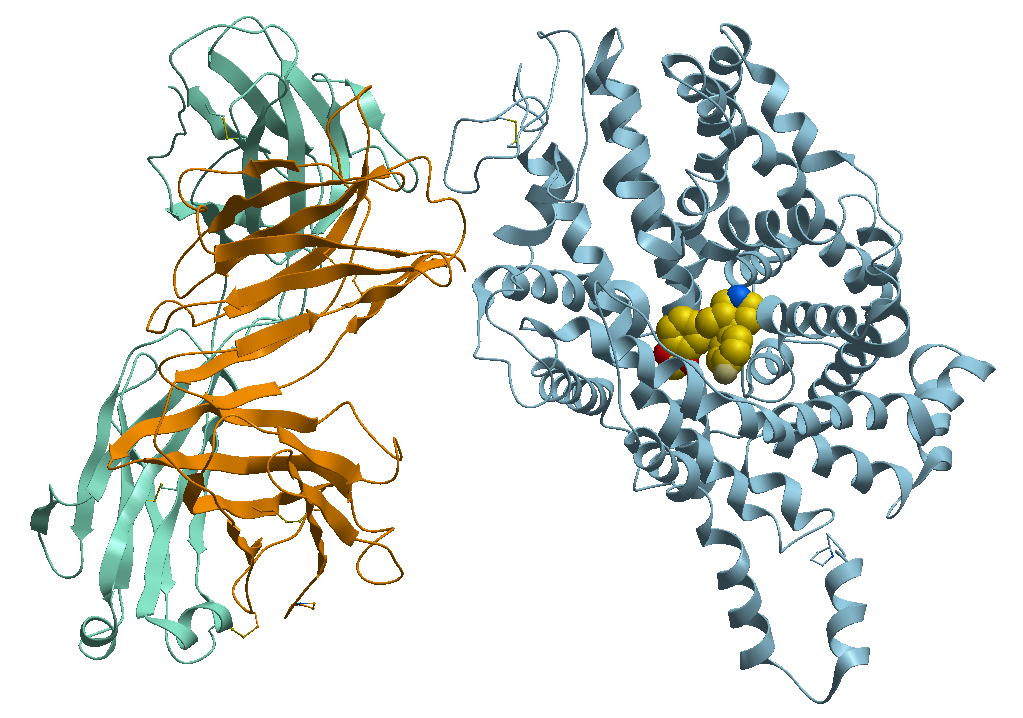
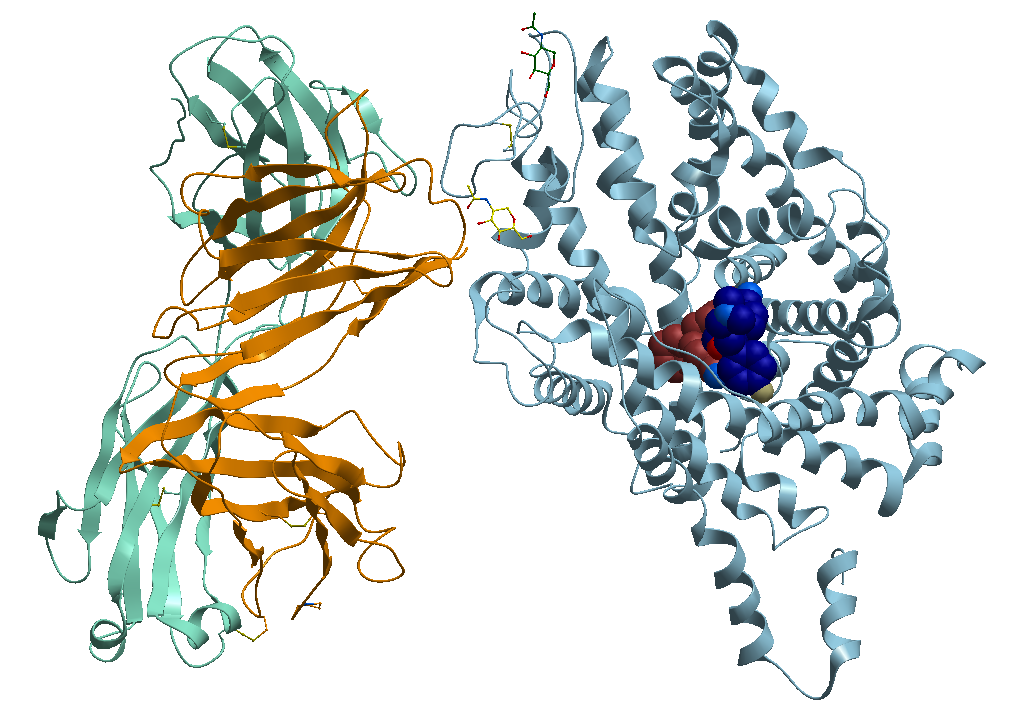
2- The TS3 Human Serotonin Transporter in bound form with the antidepressant Paroxetine known commercially as Paxil, Pexeva and Seroxat among other brands, Figure 4.
Figure 4. X-ray structure of the ts3 human serotonin transporter compl paroxetine at the central site.
https://bioinformaticstools.org/ssfs/ssfs.php?qry=5i6x
https://bioinformaticstools.org/ssfs/ssfs.php?qry=5i6x
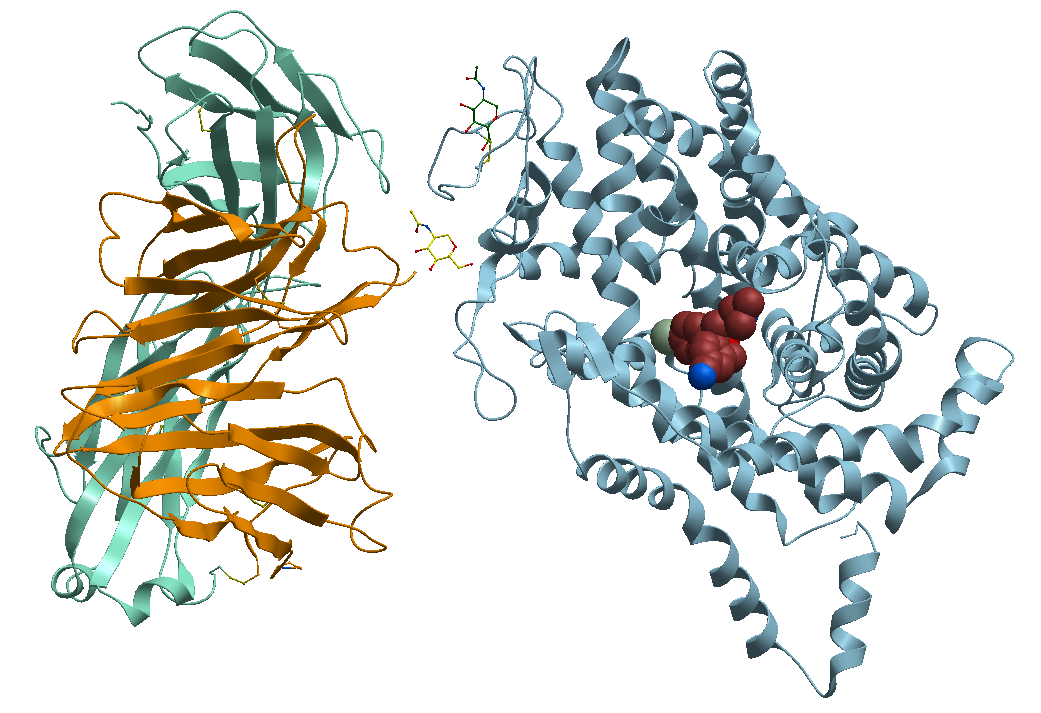
3- The TS3 Human Serotonin Transporter in bound form, at the S1, with the antidepressant S-Citalopram (the optical isomer S-(+)) known commercially as Cipralex, and Lexapro among other brand names, Figure 5.
Figure 5. X-ray structure of the ts3 human serotonin transporter compl s-citalopram at the central site.
https://bioinformaticstools.org/ssfs/ssfs.php?qry=5i71
https://bioinformaticstools.org/ssfs/ssfs.php?qry=5i71
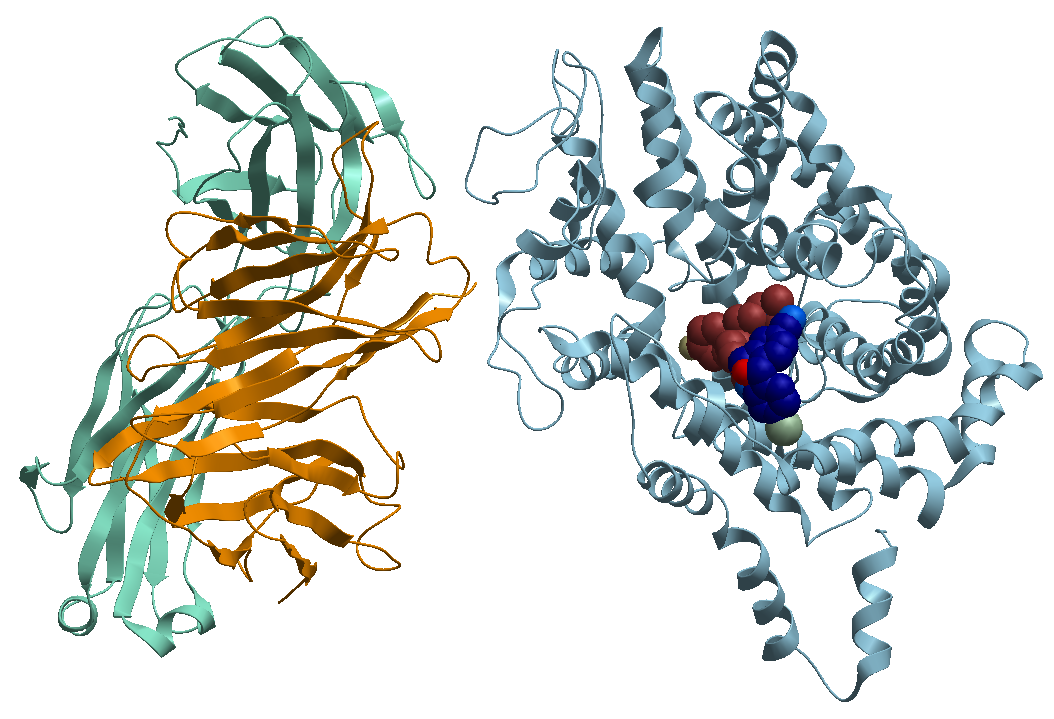
4- The TS3 Human Serotonin Transporter in bound form with the antidepressant S-Citalopram (the optical isomer S-(+)) at the two sites; the central S1 and allosteric site (S2), Figure 6.
Figure 6. X-ray structure of the ts3 human serotonin transporter compl s-citalopram at the central and allosteric sites.
https://bioinformaticstools.org/ssfs/ssfs.php?qry=5i73
https://bioinformaticstools.org/ssfs/ssfs.php?qry=5i73
5- The TS3 Human Serotonin Transporter in a bound state with a drug under study, derived from the compound S-Citalopram which bear the name Br-Citalopram, where the fluoride group has been replaced by bromine. The binding is at the central active site, Figure 7.
Figure 7. X-ray structure of the ts3 human serotonin transporter compl br-citalopram at the central site.
https://bioinformaticstools.org/ssfs/ssfs.php?qry=5i74
https://bioinformaticstools.org/ssfs/ssfs.php?qry=5i74
6- The TS3 Human Serotonin Transporter in a bound state with the drug S-Citalopram and the derivative Br-Citalopram both at the central active site, Figure 8.
Figure 8. X-ray structure of the ts3 human serotonin transporter compl s-citalopram at the central site and br-citalopram at the a site.
https://bioinformaticstools.org/ssfs/ssfs.php?qry=5i75
https://bioinformaticstools.org/ssfs/ssfs.php?qry=5i75
The antidepressants and stimulants bind to specific regions of the serotonin
According to the paper the antidepressants and amphetamines bind to specific regions of the SERT through non-covalent interactions, including hydrogen bonds, salt bridges, and pi-pi stacking interactions. These interactions occur between the chemical groups on the compound and specific amino acids in the protein, particularly those located in the binding site of the transporter.
For example, antidepressants such as fluoxetine and paroxetine bind to the S1 site of the transporter, which is located in the extracellular vestibule of the protein. In this site, the compounds interact with several amino acids, including Asp98, Asp437, and Tyr176, through hydrogen bonds and salt bridges. These interactions stabilize the binding of the compound to the protein and prevent the reuptake of serotonin into the presynaptic neuron, leading to increased levels of serotonin in the synaptic cleft.
On the other hand, amphetamines such as methamphetamine and MDMA bind to the S2 site of the transporter, which is located deeper within the protein. In this site, the compounds interact with amino acids such as Phe335, Tyr350, and Ser422 through pi-pi stacking interactions and hydrogen bonds. These interactions induce a conformational change in the protein, leading to the reverse transport of serotonin out of the presynaptic neuron and into the synaptic cleft.
The specific regions of the protein that these compounds bind to and the type of interactions involved can determine their mechanism of action and potential therapeutic effects.
Function alteration of the SERT upon antidepressants bounding
Alteration to the function of the SERT occurs upon binding the compounds such as antidepressants and amphetamines.
Once bound, these compounds can modulate the activity of the transporter in different ways.
Antidepressants such as fluoxetine and paroxetine block the reuptake of serotonin into the presynaptic neuron by binding to the S1 site of SERT. This prevents the serotonin from being removed from the synaptic cleft, leading to increased levels of serotonin in the brain. The binding of antidepressants to SERT can also induce conformational changes in the protein, which can further affect its function. For example, the binding of fluoxetine has been shown to increase the affinity of SERT for serotonin and decrease the rate of serotonin transport.
Amphetamines such as methamphetamine and MDMA, on the other hand, bind to the S2 site of SERT and induce the reverse transport of serotonin out of the presynaptic neuron and into the synaptic cleft. This causes a rapid increase in the levels of serotonin in the brain, which can lead to a feeling of euphoria. The binding of amphetamines to SERT also induces conformational changes in the protein, which can further enhance their activity. For example, the binding of methamphetamine has been shown to increase the rate of serotonin transport and decrease the affinity of SERT for serotonin.
The binding of these compounds to specific regions of SERT alters the activity of the transporter by blocking or enhancing the reuptake of serotonin, inducing conformational changes in the protein, and affecting the affinity and rate of serotonin transport. These effects can lead to changes in the levels of serotonin in the brain and have therapeutic implications for the treatment of depression and other psychiatric disorders.
X-ray structural based computational modelling and Prospects for new antidepressant drugs
The paper study describes the development of a computational model that can predict the binding of different compounds to the serotonin transporter (SERT) based on its three-dimensional structure. This model was developed using molecular dynamics simulations and docking studies, which allowed the researchers to predict the interactions between the protein and different compounds.
The computational model was based on the X-ray crystallography data of the human SERT in complex with various ligands, including antidepressants and amphetamines. The researchers used this data to create a molecular model of SERT, which was then subjected to molecular dynamics simulations to simulate the behavior of the protein in a dynamic environment.
Docking studies were then performed to predict the binding of different compounds to SERT based on their chemical structure and the interactions observed in the X-ray crystallography data. The researchers validated the computational model by comparing its predictions to experimental data on the binding of different compounds to SERT.
The computational model was shown to accurately predict the binding of different compounds to SERT, including those with high affinity for the protein such as antidepressants and amphetamines. The model can be used to design new compounds that target SERT and improve its function, as well as to predict the potential side effects of existing drugs that target the protein.
Overall, the determination of the structure and function of the SERT has important implications for our understanding of brain function and the development of new treatments for psychiatric disorders.
The development of this computational model provides a powerful tool for understanding the molecular interactions between SERT and different compounds, which can help in the development of new treatments for psychiatric disorders.
The structures can also be explored using the PDB database;
References
🕮 Coleman, J., Green, E. & Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 532, 334–339 (2016). https://doi.org/10.1038/nature17629
🕮 Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018 Apr 7;391(10128):1357-1366. doi: 10.1016/S0140-6736(17)32802-7.
🕮 Harmer CJ, Duman RS, Cowen PJ. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry. 2017 Sep;4(9):409-418. doi: 10.1016/S2215-0366(17)30135-1.