JSBB: Volume 3, Issue 1, April 2024 - STRUCTURE & FUNCTION ARTICLES,
Concept article: Review.
The Role of Bacterial Hemoglobins in Tuberculosis: Insights into Structure-Function Relationships and Drug Discovery
RACHEDI Abdelkrim📧
Departement of Biology, Faculty of Natural and Life Sciences, University of Saida-Dr. Moulay Tahar, 20000 Saida, Algeria
📧 RACHEDI Abdelkrim - E. mail: abdelkrim.rachedi@univ-saida.dz
Published: 10 April 2024
Abstract
Tuberculosis (TB) remains a significant global health threat, with millions of Mycobacterium Tuberculosis infections causing over a million of deaths annually. Despite advances in treatment, the emergence of drug-resistant strains highlights the urgent need for novel therapeutic strategies. Bacterial hemoglobins, particularly truncated hemoglobins (TrHbs), have emerged as promising targets for TB treatment due to their diverse physiological roles in stress response, virulence regulation, and metabolic adaptation. This review explores the structure-function relationships of bacterial hemoglobins and their implications for TB pathogenesis. Discussed are the recent advancements in computational modeling and protein engineering that have enhanced our understanding of bacterial hemoglobin function and regulation. Furthermore, the potential of bacterial hemoglobins are examined as targets for novel TB therapies, highlighting the challenges and opportunities associated with their development. TrHbs play crucial roles in bacterial physiology, including the response to oxidative and nitrosative stress.
Here, discussion touches upon the structural insights provided by TrHb structures from Mycobacterium Tuberculosis, represented by PDB identifiers 1s56 and 1ngk. Analysis of these structures reveals key features such as ligand-binding motifs, conformational dynamics, and functional adaptations. These findings underscore the importance of TrHbs in microbial physiology and highlight their potential as therapeutic targets against tuberculosis. Importantly, the elucidation of the structural basis of TrHb function contributed to the development of innovative strategies for combating Mycobacterium Tuberculosis infections.
Keywords
Truncated hemoglobins, Molecular dynamics, Ligand binding, Conformational changes, Allosteric regulation, Heme pocket, Oxygen binding, Nitric oxide metabolism, Oxidative stress response, Structural biology, Protein engineering.
Introduction
Tuberculosis (TB) remains a global health crisis, with an estimated 10 million new cases and 1.5 million deaths reported annually [1]. Despite significant advances in medical science, TB continues to pose formidable challenges to public health systems worldwide. The causative agent, Mycobacterium Tuberculosis, is a highly adaptable bacterium capable of evading host immune defences, establishing chronic infections, and developing resistance to conventional antibiotics [2]. This resilience is further compounded by the emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains, which render existing treatment regimens ineffective [3].
The cornerstone of TB treatment relies on a combination of antibiotics administered over a prolonged duration, typically spanning six to nine months [4]. However, the lengthy treatment duration and the emergence of drug-resistant strains pose significant hurdles to successful TB management. Patients often struggle with treatment adherence, leading to treatment failure, disease relapse, and the spread of drug-resistant strains within communities. Furthermore, the adverse side effects associated with anti-TB medications can exacerbate patient discomfort and contribute to treatment discontinuation [5].
In the context of TB pathogenesis, the interplay between Mycobacterium Tuberculosis and the host immune system is a complex and dynamic process. The bacterium employs various strategies to subvert host immune defences, evade clearance, and establish persistent infections. Central to these adaptive mechanisms are bacterial hemoglobins, particularly truncated hemoglobins (TrHbs), which play multifaceted roles in bacterial physiology and pathogenesis [6].
Bacterial hemoglobins are small heme-containing proteins that are widespread in prokaryotes, including Mycobacterium Tuberculosis. TrHbs are distinct from classical hemoglobins and possess unique structural and functional properties that enable them to thrive in diverse environmental conditions. In the context of TB, TrHbs enable Mycobacterium Tuberculosis to overcome host-imposed stresses, such as hypoxia, oxidative stress, and nitrosative stress encountered within the granulomatous lesions [7].
The significance of bacterial hemoglobins in TB pathogenesis lies in their ability to scavenge and detoxify reactive oxygen and nitrogen species generated by host immune cells. TrHbs thus neutralise these toxic compounds and help Mycobacterium Tuberculosis evade host immune surveillance, establish a replicative niche, and promote disease progression. Moreover, emerging evidence suggests that TrHbs contribute to metabolic adaptation, virulence factor production, and antibiotic tolerance, further underscoring their importance as virulence determinants [8].
Given the pivotal role of bacterial hemoglobins in TB pathogenesis, they represent promising targets for therapeutic intervention. By disrupting essential metabolic pathways, attenuating virulence factor expression, or enhancing host immune responses, inhibitors targeting TrHbs could potentially synergize with existing anti-TB drugs to improve treatment outcomes and mitigate the emergence of drug resistance. Additionally, the unique structural features of TrHbs offer opportunities for rational drug design and optimization, thereby accelerating the development of novel TB therapeutics [9].
Elucidation of the intricate interplay between bacterial hemoglobins and host factors is critical for understanding TB pathogenesis and identifying novel therapeutic targets. Research projects in the field, through harnessing the structural and functional diversity of TrHbs, can pave the way for innovative TB treatments that address the shortcomings of current therapeutic regimens and combat the global TB epidemic.
Structure-Function Relationships of Bacterial Hemoglobins
Bacterial hemoglobins, particularly truncated hemoglobins (TrHbs), exhibit a distinct structural fold around the heme group compared to their mammalian counterparts, which is intricately linked to their functional roles in microbial physiology. Unlike the three-to-three α-helical sandwich structure, Figure 1, known as globin fold, typically found in animal haemoglobin [10]. On the other hand, TrHbs often adopt a two-to-two α-helical sandwich fold, Figure 2, resulting in a more open and accessible heme pocket [11]. This structural adaptation allows for rapid ligand binding and dissociation kinetics, enabling TrHbs to efficiently scavenge and transport ligands crucial for bacterial survival and adaptation [7].
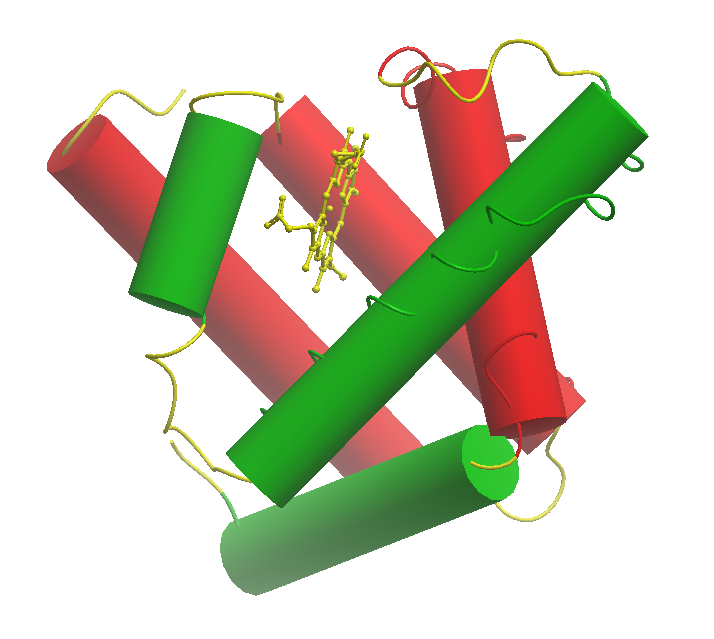
Figure1. Three-to-Three α-helical sandwich structure a model for a classical globing from sperm whale Physeter catodon
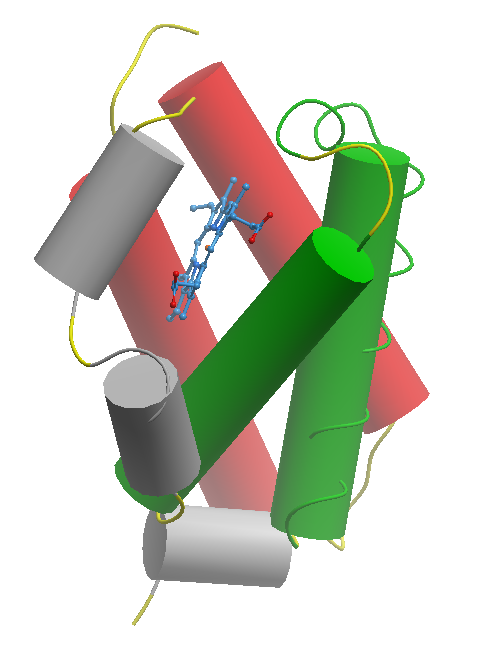
Figure2. Two-to-Two α-helical sandwich structure a model truncated haemoglobin from Mycobacterium Tuberculosis
The unique structural features of TrHbs confer specialized functions in microbial physiology, particularly in response to environmental stresses encountered within the host. For instance, studies have demonstrated the critical role of TrHbs in protecting Mycobacterium Tuberculosis from nitric oxide (NO) toxicity, a key component of the host immune response [11]. TrHbs facilitate the detoxification of this reactive species through serving as potent scavengers of NO, thereby promoting bacterial survival and persistence within the host environment [12].
The distinct structural fold of TrHbs influences their ability to modulate cellular redox balance and gene expression in response to environmental cues. Through direct interactions with transcriptional regulators or indirect modulation of signaling pathways, TrHbs contribute to the regulation of metabolic adaptation, stress response, and virulence factor production in bacterial pathogens [13]. This regulatory network enables bacteria to fine-tune their virulence phenotype in response to host-derived signals, enhancing their survival and pathogenicity [14].
Computational Modeling and Protein Engineering
In recent years, computational modeling and protein engineering have revolutionized the study of bacterial hemoglobins, offering powerful tools for elucidating structure-function relationships and unraveling their catalytic mechanisms and regulatory pathways [15]. Computational approaches, such as molecular dynamics simulations and quantum mechanical calculations, allow researchers to explore the dynamic behavior of bacterial hemoglobins at atomic resolution, providing insights into their ligand binding kinetics, allosteric regulation, and conformational dynamics.
Protein engineering techniques, including site-directed mutagenesis, directed evolution, and rational design, have facilitated the generation of tailored variants of bacterial hemoglobins with enhanced or altered functionalities [16]. Modifying key amino acid residues within the protein structure in systematic methods allowed researchers to fine-tune the ligand binding properties, stability, and catalytic activity of bacterial hemoglobins, thereby expanding their utility in biotechnological and biomedical applications [17].
Furthermore, the integration of computational modeling with experimental approaches, such as X-ray crystallography, nuclear magnetic resonance spectroscopy, and spectroscopic techniques, has enabled comprehensive structural and functional characterization of bacterial hemoglobins. These multidisciplinary approaches have provided unprecedented insights into the molecular mechanisms underlying ligand recognition, signal transduction, and allosteric regulation in bacterial hemoglobins, paving the way for the development of novel therapeutic interventions and biotechnological innovations [18].
Importance in Tuberculosis Pathogenesis
In the context of tuberculosis (TB) pathogenesis, Mycobacterium Tuberculosis encounters myriad challenges within the host environment, including oxidative and nitrosative stress imposed by the host immune system [19]. Upon infection, the bacterium is confronted with an array of host defense mechanisms aimed at eliminating the invading pathogen. Chief among these are reactive oxygen species (ROS) and reactive nitrogen species (RNS), which serve as potent antimicrobial agents capable of damaging essential biomolecules and disrupting bacterial homeostasis [20].
Bacterial hemoglobins, TrHbs, play a crucial role in mitigating the deleterious effects of oxidative and nitrosative stress on Mycobacterium Tuberculosis [6]. These hemoglobins serve as versatile scavengers of ROS and RNS, detoxifying these reactive species and protecting the bacterium from oxidative damage [7]. Moreover, recent studies have highlighted the role of bacterial hemoglobins in modulating the cellular redox balance and orchestrating adaptive responses to environmental cues [8].
Emerging evidence suggests that bacterial hemoglobins contribute to the regulation of cellular redox balance, gene expression, and virulence factor production in response to environmental cues. The process of sensing changes in oxygen tension, redox potential, and metabolic status, enable TrHbs to modulate signaling pathways that govern bacterial metabolism, stress response, and pathogenicity [21]. These regulatory functions enable Mycobacterium Tuberculosis to adapt to dynamic host environments, evade immune surveillance, and establish chronic infections.
Furthermore, bacterial hemoglobins are implicated in the regulation of gene expression and virulence factor production in Mycobacterium Tuberculosis [22]. Through direct interactions with transcriptional regulators or indirect modulation of signaling pathways, TrHbs influence the expression of genes involved in metabolism, stress response, and host-pathogen interactions [6]. This regulatory network allows the bacterium to fine-tune its virulence phenotype in response to host-derived signals, enhancing its survival and persistence within the host.
Bacterial hemoglobins play a multifaceted role in tuberculosis pathogenesis, serving as key mediators of oxidative and nitrosative stress resistance, redox homeostasis, and virulence regulation in Mycobacterium Tuberculosis. Understanding the molecular mechanisms underlying the functions of bacterial hemoglobins is essential for deciphering the pathogenesis of tuberculosis and identifying novel therapeutic targets for intervention.
Implications for Drug Discovery
The structural and functional diversity of bacterial hemoglobins presents promising opportunities for the development of innovative therapeutics against tuberculosis (TB), offering novel avenues for combating drug-resistant strains and enhancing treatment efficacy. Targeting bacterial hemoglobins, particularly tTrHbs, holds great potential for disrupting essential metabolic pathways, attenuating virulence factor production, and modulating host-pathogen interactions in Mycobacterium Tuberculosis [6].
Recent advancements in drug discovery techniques have enabled the identification and optimization of small-molecule inhibitors, peptide mimetics, and immunotherapeutic agents targeting bacterial hemoglobins. Small-molecule inhibitors designed to disrupt the heme-binding pocket or allosteric regulatory sites of TrHbs have shown promise in preclinical studies, demonstrating antimicrobial activity against drug-sensitive and drug-resistant strains of Mycobacterium Tuberculosis [11]. Additionally, peptide mimetics derived from the structural motifs of TrHbs have been developed as antimicrobial peptides, offering a novel approach to combatting TB infections [23].
Moreover, immunotherapeutic strategies aimed at targeting bacterial hemoglobins have emerged as promising adjunctive therapies for TB treatment. Stimulating host immune responses against bacterial hemoglobins can enhance immune surveillance, promote bacterial clearance, and reduce disease severity through the use of immunotherapeutic agents such as monoclonal antibodies or vaccine candidates [24]. Furthermore, combination therapies involving immunotherapeutic agents and conventional antimicrobial drugs may offer synergistic effects, enhancing treatment efficacy and reducing the emergence of drug resistance [25].
The development of innovative therapeutics targeting bacterial hemoglobins represents a paradigm shift in TB treatment, offering new opportunities to overcome the challenges associated with conventional antibiotic therapy. Researchers in the field can utilise the structural and functional properties of bacterial hemoglobins to create customized interventions. These interventions target specific virulence determinants and metabolic pathways in Mycobacterium Tuberculosis, aiming to enhance treatment efficacy and alleviate the global burden of TB [26].
Furthermore, targeting of bacterial hemoglobins for drug discovery holds significant promise for advancing TB treatment and combating drug-resistant strains. The aim of such investigation is to harness the structural and functional diversity of these proteins to innovate novel therapeutics. These therapeutics seek to provide improved efficacy and reduced toxicity, bringing renewed optimism to patients battling TB.
Online Databases for Binding Motifs Relevant to Bacterial Hemoglobins
In addition to experimental studies and structural analyses, researchers can leverage online databases to explore binding motifs relevant to bacterial hemoglobins. Online available databases include the Protein Data Bank (PDB), which contains a wealth of structural data for biomolecules, including bacterial hemoglobins. Searching the PDB helps identify available structures of bacterial hemoglobins and provide tools to analyse their ligand-binding motifs and heme-protein interactions [33, 34].
Furthermore, resources such as UniProt [35], ModBase [36], and InterPro [37] offer annotations and predictions related to ligand-binding sites and motifs in protein sequences, providing valuable insights into the structural and functional properties of bacterial hemoglobins [38, 39, 40].
More focused on structural ligands binding motifs, a relatively new online resource; the Porphyrin Proteins Binding Structural Motifs (PPBSMs) database, specializes in structural motifs that bind porphyrin groups, including those found in bacterial hemoglobins. PPBSMs is made available by the University of Saida, Algeria [41].
The PPBSMs database provides a comprehensive collection of structural motifs involved in porphyrin-protein interactions, facilitating the investigation of heme and porphyrin binding in bacterial hemoglobins. Researchers can access this database to analyse specific binding motifs, explore their structural characteristics, and gain insights into the functional significance of heme-protein interactions [41, 42].
These online databases serve as valuable tools for researchers studying bacterial hemoglobins, enabling them to explore binding motifs, analyse structural features, and uncover the functional significance of heme-protein interactions.
Discussion
The multifaceted roles of TrHbs, in tuberculosis (TB) pathogenesis underscore their significance as potential therapeutic targets for combating Mycobacterium Tuberculosis infections. The structural and functional diversity of bacterial hemoglobins enables them to perform essential physiological functions, including oxygen transport, redox homeostasis, and stress adaptation [7]. Through the exploration of the intricate connections between structure and function in bacterial hemoglobins, valuable insights into their catalytic mechanisms and regulatory pathways maybe discovered. These insights can then guide the development of precise interventions for the treatment of TB. [11].
The distinct structural characteristics of bacterial hemoglobins, such as their oligomeric state and heme-binding pocket architecture, contribute to their unique ligand-binding properties and reactivity. Truncated hemoglobins, in particular, exhibit a compact fold with a reduced number of alpha-helices, resulting in a more open heme pocket conducive to rapid ligand diffusion and binding kinetics. These structural adaptations enable bacterial hemoglobins to efficiently scavenge and transport ligands, facilitating metabolic adaptation and stress response in Mycobacterium Tuberculosis [6].
Computational modeling and protein engineering have revolutionized our understanding of bacterial hemoglobins and their roles in microbial physiology. Computational approaches, such as molecular dynamics simulations and protein structure prediction algorithms, have provided unprecedented insights into the dynamic behavior and functional dynamics of bacterial hemoglobins. Additionally, protein engineering techniques, including site-directed mutagenesis and directed evolution, have enabled the design and optimization of novel hemoglobin variants with enhanced catalytic activity and stability.
Experimental and computational tools would help uncover key determinants of bacterial hemoglobin function and regulation, shedding light on their contributions to TB pathogenesis. For example, studies have revealed the critical role of TrHbs in protecting Mycobacterium Tuberculosis from nitric oxide (NO) toxicity, a key component of the host immune response. TrHbs serve as potent scavengers of NO, detoxifying this reactive species and promoting bacterial survival within the host environment [6].
Emerging evidence suggests, moreover, that bacterial hemoglobins contribute to the regulation of gene expression and virulence factor production in Mycobacterium Tuberculosis. Through direct interactions with transcriptional regulators or indirect modulation of signaling pathways, TrHbs influence the expression of genes involved in metabolic adaptation, stress response, and host-pathogen interactions. This regulatory network enables the bacterium to fine-tune its virulence phenotype in response to host-derived signals, enhancing its survival and persistence within the host [8].
The implications of bacterial hemoglobins in TB pathogenesis extend beyond their roles in stress response and virulence regulation. These proteins represent attractive targets for the development of novel therapeutics aimed at disrupting essential metabolic pathways and attenuating bacterial virulence. Small-molecule inhibitors, peptide mimetics, and immunotherapeutic agents targeting bacterial hemoglobins offer promising avenues for TB treatment, offering synergistic effects when combined with conventional antimicrobial drugs [23].
However, several challenges remain to be addressed in the development of hemoglobin-targeted therapeutics for TB. These include optimizing the pharmacokinetic properties and bioavailability of small-molecule inhibitors, enhancing the specificity and efficacy of peptide mimetics, and elucidating the mechanisms of action underlying immunotherapeutic interventions [24]. Additionally, the potential for bacterial hemoglobin-targeted therapies to induce resistance mechanisms or unintended side effects warrants careful consideration.
The exploration of bacterial hemoglobins as therapeutic targets for TB represents a promising avenue for advancing TB treatment and combating drug resistance. The understanding of the structure-function relationships of bacterial hemoglobins, development of innovative interventions that specifically target key virulence determinants and metabolic pathways in Mycobacterium Tuberculosis are reachable, thereby improving treatment outcomes and reducing the global burden of TB.
The structures of TrHbs from Mycobacterium Tuberculosis, represented by PDB identifiers 1s56 and 1ngk, provide valuable insights into the structural and functional characteristics of these proteins [27, 29].
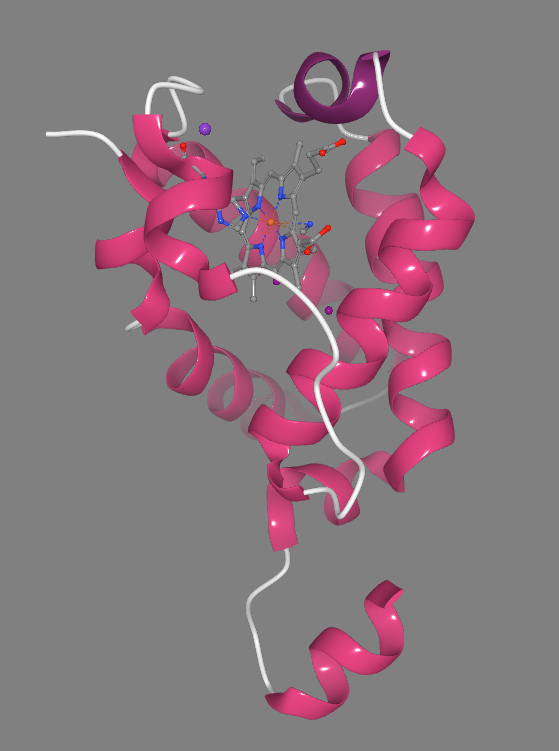
The structure with PDB identifier 1s56 reveals the three-dimensional arrangement of the TrHb protein in complex with its ligand, showcasing the binding pocket and the interactions involved in ligand recognition and stabilization [27], Figure 3. Through detailed analysis of this structure, researchers have identified key amino acid residues within the binding pocket that are crucial for ligand binding and specificity [28]. Additionally, comparison of the 1s56 structure with other TrHb structures from related bacterial species has highlighted conserved structural features and ligand-binding motifs, underscoring the functional significance of these regions in TrHb-mediated physiological processes [11].
On the other hand, the structure represented by PDB identifier 1ngk provides insights into the conformational flexibility and dynamics of TrHb proteins. The study and understanding of the structural changes that occur upon ligand binding or in response to environmental cues, this structure has contributed to our understanding of the allosteric regulation and functional adaptation of TrHbs in microbial physiology [29], Figure 4. Moreover, comparative structural analyses between ligand-bound and ligand-free states have revealed conformational changes in critical regions of the protein, shedding light on the mechanisms underlying ligand recognition and signal transduction in TrHbs [30, 31].
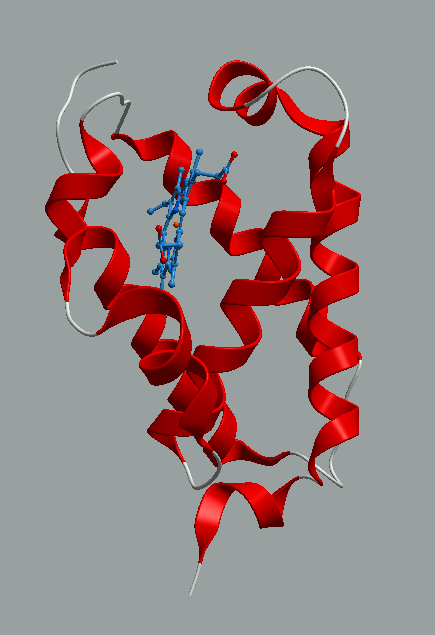
Figure4. Structure of "Truncated" Hemoglobin N from Mycobacterium Tuberculosis. Part of the conformational changes necessary in the functional mode, the bottom short helix is moved up compared to the same helix in the structure, PDB identifier 1s56 of Figure 3.
Structural analyses of TrHbs from Mycobacterium Tuberculosis, as mentioned above, PDB identifiers 1s56 and 1ngk, have provided valuable insights into the molecular mechanisms governing ligand binding, conformational dynamics, and functional adaptation of these proteins [32]. Structural data integration with biochemical and biophysical studies can facilitate continuous uncovering of the intricate relationships between structure, function, and physiological regulation in TrHbs, ultimately paving the way for the development of novel therapeutic strategies targeting bacterial hemoglobins in tuberculosis and other infectious diseases.
Conclusion
Bacterial hemoglobins represent attractive targets for the development of innovative therapeutics against TB. Knowledge and analysis of the structure-function relationships of these proteins and their roles in TB pathogenesis would lead to the identification of novel strategies to disrupt essential metabolic pathways and attenuate bacterial virulence. The structural analysis of TrHbs from Mycobacterium Tuberculosis provides valuable insights into their functional mechanisms and potential as drug targets. The identification of ligand-binding motifs and conformational dynamics enhances our understanding of TrHb-mediated responses to environmental cues. These findings offer promising opportunities for the development of novel therapeutics against tuberculosis by targeting TrHb proteins. Future research efforts should focus on further structural characterization of TrHbs and elucidation of their interactions with potential drug candidates. The study underscores the significance of structural biology in advancing our understanding of bacterial hemoglobins and their roles in infectious diseases.
The recent advancements in computational modeling and protein engineering have provided valuable insights into bacterial hemoglobin function and regulation, paving the way for the development of targeted interventions. However, the translation of these findings into effective TB therapies requires further investigation and optimization. Future research efforts should focus on overcoming the challenges associated with hemoglobin-targeted therapeutics and harnessing the full potential of bacterial hemoglobins in TB treatment. With continued interdisciplinary collaboration and technological innovation, bacterial hemoglobins hold promise as key players in the fight against TB.
Bacterial hemoglobins, TrHbs, play multifaceted roles in tuberculosis (TB) pathogenesis, contributing to stress resistance, redox homeostasis, and virulence regulation in Mycobacterium Tuberculosis. Recent advances in computational modeling and protein engineering have provided valuable insights into the structure-function relationships of TrHbs, facilitating the development of innovative therapeutics targeting these proteins. Small-molecule inhibitors, peptide mimetics, and immunotherapeutic agents targeting TrHbs hold great promise for enhancing TB treatment efficacy and combating drug resistance. Research in the field aims to transform TB treatment paradigms and alleviate the global burden of this devastating disease.
References
🕮 1. World Health Organization. Global Tuberculosis Report. Geneva, Switzerland: World Health Organization; 2021.
🕮 2. World Health Organization. Tuberculosis Fact Sheet. https://www.who.int/news-room/fact-sheets/detail/tuberculosis
🕮 3. Dheda K, Barry CE 3rd, Maartens G. Tuberculosis. Lancet. 2016;387(10024):1211-1226.
🕮 4. Nahid P, Dorman SE, Alipanah N, et al., 2016, Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin Infect Dis.63(7):e147-e195.
🕮 5. Alene KA, Yi H, Viney K, McBryde ES, Yang K, Bai L., 2017, Treatment outcomes of patients with multidrug-resistant and extensively drug resistant tuberculosis in Hunan Province, China. BMC Infect Dis.;17(1):573.
🕮 6. Sia JK, Rengarajan J. Immunology of Mycobacterium Tuberculosis Infections. Microbiol Spectr. 2019 Jul;7(4):10.1128/microbiolspec.GPP3-0022-2018. doi: 10.1128/microbiolspec.GPP3-0022-2018
🕮 7. Drvenica IT, Stančić AZ, Maslovarić IS, Trivanović DI, Ilić VL., 2022, Extracellular Hemoglobin: Modulation of Cellular Functions and Pathophysiological Effects. Biomolecules. Nov 17;12(11):1708. doi: 10.3390/biom12111708.
🕮 8. Kumar A, Farhana A, Guidry L, Saini V, Hondalus M, Steyn AJ., 2011, Redox homeostasis in mycobacteria: the key to tuberculosis control? Expert Rev Mol Med. Dec 16;13:e39. doi: 10.1017/S1462399411002079
🕮 9. Zumft WG., 1997, Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev.;61(4):533-616.
🕮 10. Katia Marino., 2008, Expression, isolation and characterisation of tetrameric haemoglobin as a model to study the Root effect, Ph.D. Thesis, University of Naples “Federico II”
🕮 11. Ouellet H, Ouellet Y, Richard C, Labarre M, Wittenberg B, Wittenberg J, Guertin M., 2002, Truncated hemoglobin HbN protects Mycobacterium bovis from nitric oxide. Proc Natl Acad Sci U S A. Apr 30;99(9):5902-7
🕮 12. Bolognesi M, Bordo D, Rizzi M, Tarricone C, Ascenzi P., 1997, Nonvertebrate hemoglobins: structural bases for reactivity. Prog Biophys Mol Biol.;68(1):29-68.
🕮 13. Johannes F. Coy, Zdenek Sedlacek, Dietmar Bächner, Hajo Delius, Annemarie Poustka,. 1999. A Complex Pattern of Evolutionary Conservation and Alternative Polyadenylation Within the Long 3′-Untranslated Region of the Methyl-CpG-Binding Protein 2 Gene (MeCP2) Suggests a Regulatory Role in Gene Expression, Human Molecular Genetics, Volume 8, Issue 7, July, Pages 1253–1262
🕮 14. Green J, Rolfe MD, Smith LJ., 2014, Transcriptional regulation of bacterial virulence gene expression by molecular oxygen and nitric oxide. Virulence.;5(8):794-809. doi: 10.4161/viru.27794.
🕮 15. Couture M, Yeh SR, Wittenberg BA, Wittenberg JB, Ouellet Y, Rousseau DL, Guertin M., 1999, A cooperative oxygen-binding hemoglobin from Mycobacterium Tuberculosis. Proc Natl Acad Sci U S A. Sep 28;96(20):11223-8. doi: 10.1073/pnas.96.20.11223.
🕮 16. Raman CS, Li H, Martásek P, Král V, Masters BS, Poulos TL., 1998, Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell. 1998;95(7):939-950.
🕮 17. Yeh SR, Couture M, Ouellet Y, Guertin M, Rousseau DL., 2000, A cooperative oxygen binding hemoglobin from Mycobacterium Tuberculosis. Stabilization of heme ligands by a distal tyrosine residue. J Biol Chem. Jan 21;275(3):1679-84. doi: 10.1074/jbc.275.3.1679.
🕮 18. Geoghegan KF, Dixon HB, Rosner PJ, et al., 1999, Spontaneous alpha-N-6-phosphogluconoylation of a "His tag" in Escherichia coli: the cause of extra mass of 258 or 178 Da in fusion proteins. Anal Biochem.; 267(1):169-184.
🕮 19. Ouellet H, Ouellet Y, Richard C, et al., 2002, Truncated hemoglobin HbN protects Mycobacterium bovis from nitric oxide. Proc Natl Acad Sci U S A.;99(9):5902-5907.
🕮 20. MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, Chen H, Mudgett JS., 1995, Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell.;81(4):641-650.
🕮 21. Sarti P, Giuffrè A, Forte E, Mastronicola D, Barone MC, Brunori M., 2000, Nitric oxide and cytochrome c oxidase: mechanisms of inhibition and NO degradation. Biochem Biophys Res Commun.;274(1):183-187.
🕮 22. Reynolds C. M., Meyer J., and Poole L. B., 2000, An NADH-Dependent Bacterial Thioredoxin Reductase-like Protein in Conjunction with a Glutaredoxin Homologue Form a Unique Peroxiredoxin (AhpC) Reducing System in Clostridium pasteurianum. Biochemistry 2002 41 (6), 1990-2001
🕮 23. Oliveira GS, Costa RP, Gomes P, Gomes MS, Silva T, Teixeira C., 2021, Antimicrobial Peptides as Potential Anti-Tubercular Leads: A Concise Review. Pharmaceuticals (Basel). Apr 2;14(4):323. doi: 10.3390/ph14040323.
🕮 24. Achkar JM, Prados-Rosales R., 2018, Updates on antibody functions in Mycobacterium Tuberculosis infection and their relevance for developing a vaccine against tuberculosis. Curr Opin Immunol.;53:30-37.
🕮 25. Jindani A, Harrison TS, Nunn AJ, Phillips PPJ, Churchyard GJ, Charalambous S, Hatherill M, Geldenhuys H, McIlleron HM, Zvada SP, Mungofa S, Shah NA, Zizhou S, Magweta L, Shepherd J, Nyirenda S, van Dijk JH, Clouting HE, Coleman D, Bateson ALE, McHugh TD, Butcher PD, Mitchison DA., 2014, High-dose rifapentine with moxifloxacin for pulmonary tuberculosis. N Engl J Med.;371(17):1599-1608.
🕮 26. Zumla A, Rao M, Parida SK, Keshavjee S, Cassell G, Wallis R, Axelsson-Robertsson R, Doherty M, Andersson J, Maeurer M., 2015, Inflammation and tuberculosis: host-directed therapies. J Intern Med.;277(4):373-387.
🕮 27. Milani M, Pesce A, Ouellet Y, Dewilde S, Friedman J, Ascenzi P, Guertin M, Bolognesi M., 2004, Heme-ligand tunneling in group I truncated hemoglobins. J Biol Chem. May 14;279(20):21520-5. doi: 10.1074/jbc.M401320200.
🕮 28. Geuens E, Brouns I, Flamez D, Dewilde S, Timmermans JP, Moens L., 2003, A globin in the nucleus! J Biol Chem.;278(32):30417-30420.
🕮 29. Milani M, Savard PY, Ouellet H, Ascenzi P, Guertin M, Bolognesi M., 2003, A TyrCD1/TrpG8 hydrogen bond network and a TyrB10TyrCD1 covalent link shape the heme distal site of Mycobacterium Tuberculosis hemoglobin O. Proc Natl Acad Sci U S A. May 13;100(10):5766-71. doi: 10.1073/pnas.1037676100.
🕮 30. Samuni U, Dantsker D, Ray A, Wittenberg JB, Wittenberg BA, Dewilde S, Moens L, Ouellet Y, Guertin M, Friedman JM., 2003, Kinetic modulation in carbonmonoxy derivatives of truncated hemoglobins: the role of distal heme pocket residues and extended apolar tunnel. J Biol Chem. Jul 18;278(29):27241-50. doi: 10.1074/jbc.M212634200.
🕮 31. Milani M, Pesce A, Nardini M, Ouellet H, Ouellet Y, Dewilde S, Bocedi A, Ascenzi P, Guertin M, Moens L, Friedman JM, Wittenberg JB, Bolognesi M., 2005, Structural bases for heme binding and diatomic ligand recognition in truncated hemoglobins. J Inorg Biochem. Jan;99(1):97-109. doi: 10.1016/j.jinorgbio.2004.10.035.
🕮 32. Wittenberg JB, Bolognesi M, Wittenberg BA, Guertin M., 2002, Truncated hemoglobins: a new family of hemoglobins widely distributed in bacteria, unicellular eukaryotes, and plants. J Biol Chem. Jan 11;277(2):871-4. doi: 10.1074/jbc.R100058200. Epub 2001 Nov 5.
🕮 33. Protein Data Bank (PDB). Available online: https://www.rcsb.org/
🕮 34. Berman, H. M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T. N., Weissig, H., ... & Bourne, P. E., 2000, The Protein Data Bank. Nucleic acids research, 28(1), 235-242
🕮 35. UniProt. Available online: https://www.uniprot.org/
🕮 36. ModBase. Available online: https://modbase.compbio.ucsf.edu/
🕮 37. InterPro. Available online: https://www.ebi.ac.uk/interpro/
🕮 38. The UniProt Consortium, UniProt: the Universal Protein Knowledgebase in 2023, 2023, Nucleic Acids Research, Volume 51, Issue D1, Pages D523–D531, https://doi.org/10.1093/nar/gkac1052
🕮 39. Pieper U, Eswar N, Davis FP, Braberg H, Madhusudhan MS, Rossi A, Marti-Renom M, Karchin R, Webb BM, Eramian D, Shen MY, Kelly L, Melo F, Sali A., 2006, MODBASE: a database of annotated comparative protein structure models and associated resources. Nucleic Acids Res. 1;34 (Database issue):D291-5. doi: 10.1093/nar/gkj059
🕮 40. Paysan-Lafosse T, Blum M, Chuguransky S, Grego T, Pinto BL, Salazar GA, Bileschi ML, Bork P, Bridge A, Colwell L, Gough J, Haft DH, Letunić I, Marchler-Bauer A, Mi H, Natale DA, Orengo CA, Pandurangan AP, Rivoire C, Sigrist CJA, Sillitoe I, Thanki N, Thomas PD, Tosatto SCE, Wu CH, Bateman A., 2023, InterPro in 2022, Nucleic Acids Research, doi: 10.1093/nar/gkac993
🕮 41. PPBSMs - Porphyrin Proteins Binding Structural Motifs. Available online: https://bioinformatics.univ-saida.dz/prjs/ppbsms/
🕮 42. Megharbi M. El., Bitar M. and Rachedi A., 2023, Structural and Functional Binding Motifs in Porphyrin Proteins: Insights into Ligands and Biological Function, JSBB, Vol. 2, Issue 2, August 2023. https://bioinformatics.univ-saida.dz/jsbb/August_2023/Porphyrin_Proteins_Binding_Structural_Motifs.pdf