JSBB: Volume 1, Issue 4, March 2023
Concept article: Minireviews - STRUCTURE & FUNCTION ARTICLES
Ribozymes; Structure, Function and Therapeutic applications
RACHEDI Abdelkrim
Laboratory of Biotoxicology, Pharmacognosy and biological valorisation of plants, Faculty of Sciences, Department of Biology, University of Saida - Dr Moulay Tahar, 20100 Saida, Algeria.
📧 E. mail: abdelkrim.rachedi@univ-saida.dz
Published: 01 March 2023
Abstract
Ribozymes are RNA molecules capable of catalysing chemical reactions. These molecules play an important role in
a variety of biological processes such as gene expression, RNA processing, and viral replication.
The discovery of ribozymes has had a profound impact on our understanding of biology and has challenged long-held assumptions about the exclusive role of proteins in catalysing chemical reactions. Advances in our understanding of ribozymes have led to the development of new technologies and therapeutics, and the study of ribozymes continues to be an active area of research in molecular biology.
In this article, we will discuss the different types of ribozymes, their structures, and their functions. It also provides a number of examples
of ribozymes diversity as catalytic RNA molecules and their roles in various biological systems. The article also implementations of ribozymes in medicine and as a tool that provide enhenced
precision in CRISPR/Cas9 targeting.
Key words
RNA, Ribozymes, Enzymes, Proteins, CRISPR, Therapeutic applications, Biological Function, 3D-Structure
Introduction
A milestone discovery, in biology, that marked the end of the 20th century (the 1980s) was the discovery that RNA can do / mediate enzymes
like biochemical reactions. These have gone with the name Ribozymes✯ that is to mean 'RNA based enzymes' this is in contrast with the long
held belief that enzymes are or should be protein based entities.
Ribozymes are RNA molecules that have the ability to catalyze chemical reactions. Unlike traditional enzymes, which are typically proteins,
ribozymes are made up of RNA. The discovery of ribozymes challenged the long-held belief that proteins were the only molecules capable of
catalyzing reactions. Ribozymes are now known to play important roles in many biological processes, including gene expression, RNA processing,
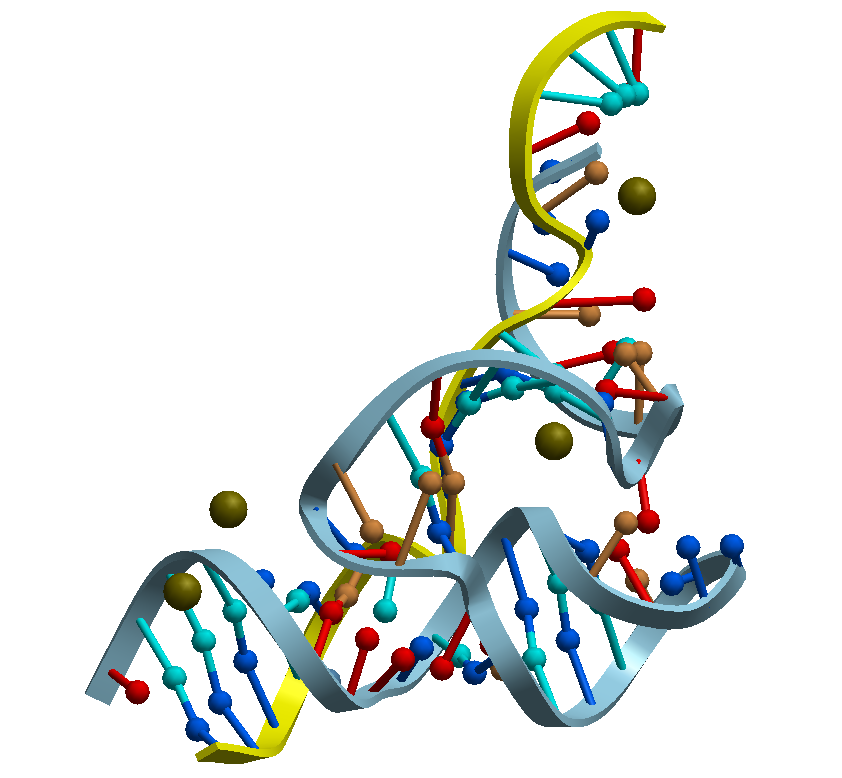
and viral replication, Figure 1.
Figure 1. HammerHead Ribozyme
https://bioinformatics.univ-saida.dz/ssfs/ssfs.php?qry=5dqk
https://bioinformatics.univ-saida.dz/ssfs/ssfs.php?qry=5dqk
Ribozymes were first associated with plant's viruses and sub-viral agents (viral satellites) but more studies (2010 onward) showed ribozymes
linked to many species' genomes including Humans, some are found linked to non-coding regions like introns!, refer to Methods and Discussion sections.
Different venues and methods of research are on going to try and understand the biological roles and functions of ribozymes including
structural studies which revealed their 3D-structure; which lead steps closer to understanding their modes of action and related applications, image below.
The images first and second, attached below, depict the 3D-structure of the HammerHead Ribozyme. Details of the structure and function
of an examples of a HammerHead Ribozyme can be explored using the SSFS⭕ application on the bioinformatics site, Department of Biology,
Univ. of Saida - Algeria, through the link: https://bioinformatics.univ-saida.dz/ssfs/ssfs.php?qry=5dqk (see Figure 2).
The structure can also be explored at the European PDB site: pdbe.org/5dqk
Figure 2. Two Divalent Metal Ions And Conformational Changes Play Role Hammerhead Ribozyme Cleavage Reaction-Wt Ribozyme In Mg2+
Find details on the SSFS web-tool: https://bioinformatics.univ-saida.dz/ssfs/ssfs.php?qry=5dqk
Find details on the SSFS web-tool: https://bioinformatics.univ-saida.dz/ssfs/ssfs.php?qry=5dqk
It is worth mentioning that even Ribosomes and tRNA molecules can be considered as kinds of more complex types
of ribozymes involved in the vital roles of translating the genetic code into biologically functional entities
and hence involved in life itself.
Ribozymes pose lots of other questions including those about the origins of life and the genetic code; whether
the RNA or DNA was the first (life) information making and storing molecular system; the RNA World Hypothesis.
⭕ SSFS: Sequence, Structure and Function Server by the Structural Biology & Bioinformatics Groups, Biology Dept, Faculty of Science, University of Saida, Algeria
نظام: التركيب الأولي، التركيب الفراغي و الوظيفة البيولوجية ، فريق البيولوجيا ثلاثية
الأبعاد و المعلوماتية الحيوية، قسم البيولوجيا، كلية العلوم، جامعة سعيدة، الجزائر
Methods
One of the earliest methods used to identify ribozymes was the selection of RNA molecules that were capable of
catalysing chemical reactions in vitro. In 1986, Thomas Cech and his colleagues discovered a ribozyme that could
catalyse the splicing of its own precursor RNA in Tetrahymena thermophila, a type of ciliated protozoan.
Another important method used to identify ribozymes was the analysis of RNA molecules that were involved in RNA
processing. Group I and II introns were first identified as self-splicing RNA molecules that were capable of
catalysing their own splicing. Later, RNase P was identified as an RNA molecule that catalyses the cleavage
of precursor tRNA molecules. In 1989, Sidney Altman and his colleagues discovered that RNase P was actually
a ribonucleoprotein complex that contained a catalytic RNA subunit.
To understand the structure and function of ribozymes, scientists have used a variety of techniques
including X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and computational modeling.
These methods have provided insights into the complex structures and mechanisms of ribozymes.
Discussion
Ribozymes can be divided into two main categories: self-cleaving ribozymes and catalytic RNA. Self-cleaving ribozymes
are capable of catalyzing their own cleavage and include the hammerhead, hairpin, and hepatitis delta virus (HDV) ribozymes.
Catalytic RNA, on the other hand, catalyzes reactions involving other molecules and includes the group I and group II introns,
RNase P, and the telomerase RNA component.
One example of a ribozyme is the hammerhead ribozyme, which is found in some plant viruses. The hammerhead ribozyme
catalyzes the cleavage of the viral RNA, which is necessary for the virus to replicate.
Another example is the group
I intron, which is found in some bacteria and fungi. The group I intron catalyzes its own splicing from the precursor
RNA molecule, allowing for proper gene expression.
The catalytic mechanisms of ribozymes can vary, but they generally involve the formation of a specific three-dimensional
structure that brings the reactive groups into close proximity. This structure is often stabilized by interactions between
complementary base pairs.
Diversity of catalytic RNA molecules in Ribozymes
RNase P:
This ribozyme was originally thought to be a protein, but it was later discovered that the catalytic activity of RNase P is actually carried out by an RNA molecule. RNase P cleaves precursor tRNA molecules at their 5' ends to produce the mature tRNA. This cleavage is essential for the proper folding and function of the tRNA, which is necessary for protein synthesis. RNase P is found in all domains of life and has a conserved RNA subunit, indicating that it likely originated early in the evolution of life.
Group I and II introns:
These ribozymes are found in some genes and are capable of catalyzing their own splicing. Group I introns are found in some genes of bacteria, mitochondria, and chloroplasts, while Group II introns are found in bacteria, archaea, and eukaryotes. Both types of introns can catalyze their own splicing by using a variety of chemical mechanisms. These ribozymes were some of the first examples of catalytic RNA molecules to be discovered.
Hammerhead ribozyme:
The hammerhead ribozyme is a small RNA molecule that is capable of cleaving RNA molecules at specific sites. This ribozyme was originally discovered in the RNA genome of a small satellite virus that infects plants. The hammerhead ribozyme has been used as a model system for studying RNA catalysis and has also been used in the development of new gene therapy techniques.
Hairpin ribozyme:
The hairpin ribozyme is another small RNA molecule that is capable of catalyzing the cleavage of RNA molecules. This ribozyme was discovered in the RNA genome of a plant viroid, which is a small, circular RNA molecule that is capable of replicating in plant cells. The hairpin ribozyme has been used in a variety of applications, including the development of RNA sensors and the design of artificial RNA switches.
Peptidyl transferase 23S rRNA:
This ribozyme is found in the large subunit of the bacterial ribosome and catalyzes the formation of peptide bonds between amino acids during protein synthesis. The 23S rRNA component of the ribosome has been shown to have a key role in the catalysis of peptidyl transferase activity. This ribozyme is essential for bacterial survival and has been a target for the development of antibiotics.
GIR1 branching ribozyme:
This ribozyme was discovered in the genome of the plant Arabidopsis thaliana and is capable of catalyzing the branching of RNA molecules. This ribozyme has a unique structure and mechanism of action compared to other known ribozymes, and its discovery has provided new insights into the diversity of catalytic RNA molecules in nature.
Leadzyme:
The leadzyme is a ribozyme that is capable of specifically binding to and cleaving lead ions, which are toxic to living organisms. The leadzyme was originally discovered in the RNA genome of a bacteriophage and has been used in the development of new biosensors for detecting lead contamination in the environment.
HDV ribozyme:
The hepatitis delta virus (HDV) ribozyme is a self-cleaving RNA molecule that is essential for the replication of the HDV genome. This ribozyme is unique in that it requires the presence of a specific protein for its activity. The HDV ribozyme has been used as a model system for studying RNA-protein interactions and has also been explored as a potential target for antiviral therapies.
VS ribozyme:
The VS ribozyme is a small RNA molecule that is capable of cleaving RNA molecules at specific sites. This ribozyme was discovered in the genome of a plant virus and has been used in the development of new gene therapy techniques.
Mammalian CPEB3 ribozyme:
The CPEB3 ribozyme is found in mammals and is involved in regulating the translation of messenger RNA (mRNA) molecules. The CPEB3 ribozyme is located in the 3' untranslated region (UTR) of certain mRNAs and can cleave itself in response to specific signals, leading to changes in mRNA translation. This ribozyme has been implicated in a variety of cellular processes, including synaptic plasticity and memory formation.
CoTC ribozyme:
The CoTC ribozyme is a ribozyme that is found in bacteria and is involved in the biosynthesis of coenzyme B12. This ribozyme catalyzes the cleavage of an RNA molecule that is necessary for the formation of the cofactor. The CoTC ribozyme has been studied as a potential target for antibacterial therapies.
glmS ribozyme:
The glmS ribozyme is a ribozyme that is found in some bacteria and is involved in the regulation of gene expression. This ribozyme is located in the 5' UTR of the mRNA for the enzyme responsible for the biosynthesis of glucosamine-6-phosphate, which is an essential precursor for the synthesis of peptidoglycan, a component of bacterial cell walls. The glmS ribozyme can cleave itself in the presence of glucosamine-6-phosphate, leading to a decrease in the expression of the enzyme and a reduction in peptidoglycan synthesis. The glmS ribozyme has been explored as a potential target for antibacterial therapies.
These examples of ribozymes highlight the diversity of catalytic RNA molecules that exist in nature and their importance in a variety of biological processes. The study of ribozymes has provided insights into the evolution of life on Earth and has also led to the development of new technologies and therapies.
Medical Implementations of Ribozymes
HIV therapy:
Researchers have developed a ribozyme-based therapy for HIV, which targets the virus's RNA genome and cleaves it, preventing it from replicating. Clinical trials have shown promising results, with a reduction in viral load and an increase in CD4+ T-cell count.
Cancer therapy:
Ribozymes have been designed to target cancer-causing genes, such as the BCR-ABL fusion gene in chronic myelogenous leukemia. In preclinical studies, these ribozymes have shown efficacy in reducing tumor growth and prolonging survival.
Antisense therapy:
Ribozymes can be combined with antisense oligonucleotides to target specific mRNA molecules, cleaving them and preventing translation into proteins. This approach has been used to target disease-causing proteins, such as in the case of Duchenne muscular dystrophy.
Gene therapy:
Ribozymes have been used as a tool for gene therapy, where they can be used to cleave specific mRNA molecules, leading to the downregulation or knockdown of a target gene. This approach has shown promise in treating inherited diseases such as Huntington's disease.
Ribozyme-mediated CRISPR/Cas9
This is a type of gene editing technique that involves the use of ribozymes to improve the specificity and
accuracy of the CRISPR/Cas9 system. Ribozymes are used to specifically target the Cas9 nuclease to the site of interest, where it can then cleave the
DNA and introduce desired modifications.
There are currently several studies exploring the use of ribozymes in combination with the CRISPR/Cas9 system.
A number of studies demonstrated that the use of ribozymes improved the specificity and efficiency of the CRISPR/Cas9 system, reducing off-target effects
and increasing the precision of genome editing. Other studies showed that ribozyme-mediated CRISPR/Cas9 could be used to effectively correct genetic
mutations associated with cystic fibrosis.
However, more research is needed to fully evaluate the potential of ribozyme-mediated CRISPR/Cas9 and its applicability to various gene editing applications.
Conclusion
Ribozymes are important molecules that play a crucial role in many biological processes. They provide insight into the diversity
and complexity of biological molecules and challenge the long-held belief that proteins are the only molecules capable of
catalyzing chemical reactions. Advances in our understanding of ribozymes will continue to inform the development of new
therapeutics and technologies.
References
🕮 Cech, T. R. (1986). The generality of self-splicing RNA: relationship to nuclear mRNA splicing. Cell, 44(2), 207-210.
🕮 Cech, T. R., & Steitz, J. A. (2014). The noncoding RNA revolution—trashing old rules to forge new ones. Cell, 157(1), 77-94.
🕮 Coppins, R. L., & Hall, K. B. (2017). Grooving to the tune of ribozyme catalysis. RNA, 23(4), 471-476.
🕮 Fedonina, A. S., & Cech, T. R. (2016). Ribozymes: exploring RNA catalysts. ACS Chemical Biology, 11(3), 571-581.
🕮 Gambill, L., Staubus, A., Mo, K.W., Ameruoso, A. & Chappell, J. (2023). A split ribozyme that links detection of a native RNA to orthogonal protein outputs. Nature Communications, 14(543).
🕮 Kruger, K., Grabowski, P. J., Zaug, A. J., Sands, J., Gottschling, D. E., & Cech, T. R. (1982). Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell, 31(1), 147-157.
🕮 James, H. A., Gibson, A. (1998). The Therapeutic Potential of Ribozymes. Blood, 91(2): 371–382 .https://doi.org/10.1182/blood.V91.2.371
🕮 Li, JW., Zeng, T., Xu, ZZ., Li, JJ., Hu, H., Yu, Q., Zhou, L., Zheng, RR., Luo, J. & Wang, CY. (2022). Ribozyme-mediated CRISPR/Cas9 gene editing in pyrethrum (Tanacetum cinerariifolium) hairy roots using a RNA polymerase II-dependent promoter. Plant Methods, 18(32)
🕮 Liu, Y., & Wilson, T. J. (2016). Ribozymes as potential tools for therapeutics and insights into biological chemistry. Frontiers in Chemistry, 4, 21.
🕮 Mir, A., Chen, J., Robinson, K., Lendy, E, Goodman, J., Neau, D., Golden, B.L. (2015). Two Divalent Metal Ions and Conformational Changes Play Roles in the Hammerhead Ribozyme Cleavage Reaction. Biochemistry, 54(41), 6369-81
🕮 Pyle, A. M. (2016). Ribozymes: a distinct class of metalloenzymes. Science, 352(6293), 1177-1180.
🕮 Shang, R., Zhang, F., Xu, B., Xi, H., Zhang, X., Wang, W., & Wu, L. (2015). Ribozyme-enhanced single-stranded Ago2-processed interfering RNA triggers efficient gene silencing with fewer off-target effects. Nature Communications, 6(8430). . https://doi.org/10.1038/ncomms9430
🕮 Strobel, S. A. (2017). Ribozymes. Current Biology, 27(13), R698-R699.
🕮 Szostak, J. W., Bartel, D. P., & Luisi, P. L. (2001). Synthesizing life. Nature, 409(6818), 387-390.
🕮 Taha Ahmed, B.,& Abdelkrim, R. (2022). CRISPR-Cas9: a powerful and precise genomic editing tool. JSBB., 1(3). CRISPR_powerful_precise_genome_edtn