JSBB: Volume 2, Issue 4, February 2024 - BIOTECHNOLOGY ARTICLES
Research article: Master's research based.
This paper is related to a project Master’s degree in Biological Sciences Startup Diploma 2022-2023, within the framework of the Ministerial Decree 1275.
هذا المقال حول مشروع ماستر 2022-2023 لنيل شهادة مؤسسة ناشئة في إطار القرار الوزاري 1275
Used cooking oil as a renewable and cost-effective resource for biodiesel production in Algeria
CHOHRA Younes1, BOUKHALOUA Oussama1, GHELLAI Lotfi1,2,📧
1 Departement of Biology, Faculty of Natural and Life Sciences, University of Saida-Dr. Moulay Tahar, 20000 Saida, Algeria
2 Laboratory of Biotoxicology, Pharmacognosy and biological valorisation of plants Compus Ain El Hadjar, Faculty of Sciences, Department of Biology, University of Saida Dr Tahar Moulay, 20100 Saida, Algeria.
📧 GHELLAI Lotfi - E. mail: lotfi.ghellai@univ-saida.dz, lotfi.ghellai@hotmail.ch
Published: 25 February 2024
Abstract
Due to the awareness of the negative impact of conventional fuels on the environment and the frequent rise in crude oil prices, the need for sustainable and environmentally friendly alternative energy sources has become increasingly important in recent years. The aim of this study is to investigate the production of biodiesel from used cooking oil (UCO) as a sustainable and renewable fuel source. The study provides an overview of the biodiesel production process, including the transesterification method, which involves the reaction of UCO with alcohol and a catalyst to produce biodiesel. The properties and characteristics of biodiesel are also examined. Overall, this study aims to contribute to the growing body of knowledge on biodiesel production from UCO and provide valuable insights into its feasibility, benefits and potential applications.
Key words
Biodiesel, Petrodiesel, fuel, Glycerol, Transesterification, Used cooking oil, Waste management.
Introduction
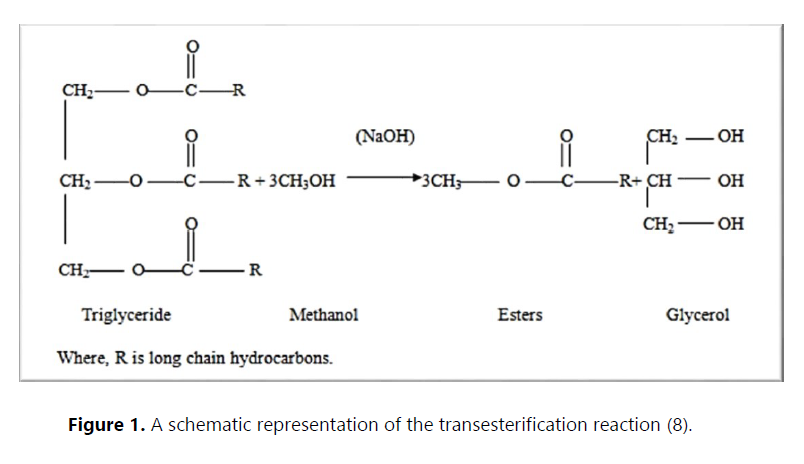
The 21st century faces many issues, such as energy sustainability, environmental problems and rising fuel prices. Conventional fuels are known to pollute the air with emissions of sulfur dioxides, carbon dioxide, particulate matter and other gases. This has led to increased research into alternative fuels and renewable energy sources (1). Renewable energies can be seen as alternatives to fossil fuels. They are already being used in many countries around the world. Biomass is the most widespread form of renewable energy and is the most important source of primary energy supply among the renewable forms of energy. Renewable resources account for around 10% of global energy consumption and can be converted into other usable forms of energy such as biofuels.Among biofuels, biodiesel is one of the possible alternatives in the field of transportation. Biodiesel is the name given to a variety of ester-based oxygenated fuels from renewable biological sources. Chemically, biodiesel is defined as a monoalkyl ester of long-chain fatty acids from renewable biolipids(2). Biodiesel can be produced from a variety of feedstocks. These feedstocks include the most common vegetable oils (e.g. soybean, cottonseed, palm, peanut, rapeseed, sunflower, safflower, coconut) and animal fats (usually tallow), as well as used cooking oils (UCO), also known as waste oil. Repeated frying for preparation of food makes the edible vegetable oil unsuitable for consumption due to high free fatty acid (FFA) content(3). UCO is associated with numerous disposal problems, such as water and soil pollution, human health concern and disturbance to the aquatic ecosystem(3; 4). Instead of discarding it and harming the environment, it can be used as an effective and cost-efficient feedstock for biodiesel production as it is readily available (5). The conversion of UCO into biodiesel through the transesterification process (Figure 1) reduces the molecular weight to one-third, reduces the viscosity by about one-seventh, reduces the flash point slightly, increases the volatility marginally and reduces pour point considerably (6). Then, the fuel produced has approximately the same property of petrodiesel and can be used in conventional diesel engines without any change in this last.
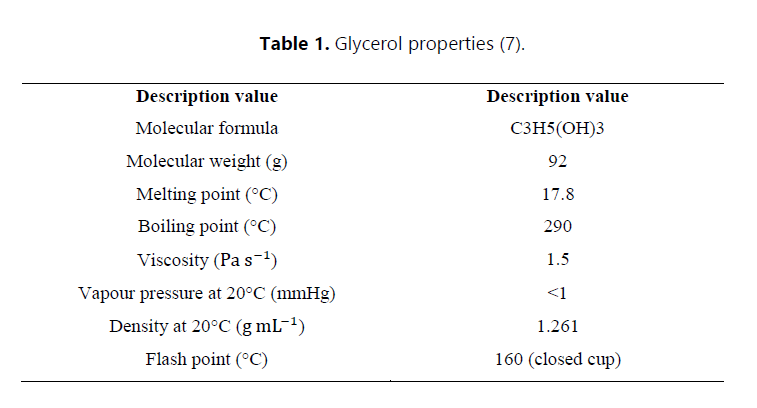
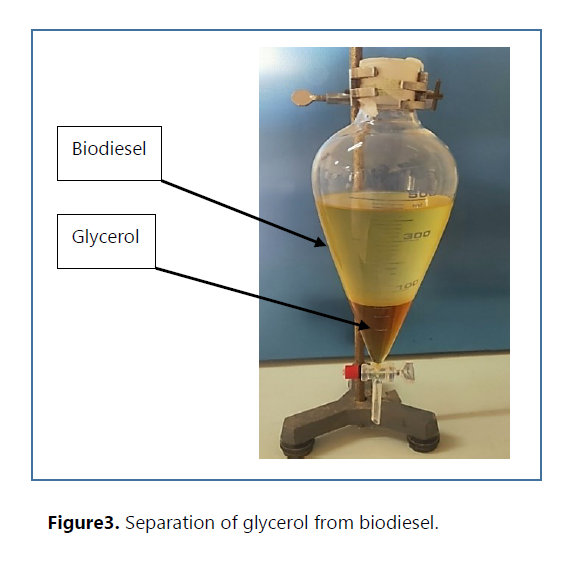
Furthermore, the production process yields a valuable byproduct, glycerol. This can be recovered and purified using a variety of practical techniques and methods such as centrifuging, bleaching and chemical treatment. Glycerol (Table 1) can be used in various industries such as food, cosmetics and pharmaceuticals. It has many valuable properties, including being a good moisturizer, emollient, plasticizer, thickener, solvent, dispersing medium, lubricant, sweetener and an antifreeze agent (7).
Biodiesel can be used directly as a fuel in diesel engines -in cars, busses, trucks and boats - without the need for engine modifications. Its physical properties are similar to those of petroleum diesel.Biodiesel can be used alone (B100) or blended with petroleum diesel in many different concentrations, e.g. B100 (pure biodiesel), B20 (20% biodiesel, 80% petroleum diesel), B5 (5% biodiesel, 95% petroleum diesel) and B2 (2% biodiesel, 98% petroleum diesel). The advantages of biodiesel as a diesel fuel are its higher biodegradability, higher combustion efficiency and lower emissions compared to petrodiesel. Biodiesel is non-toxic and degrades about four times faster than petrodiesel. Its oxygen content improves the biodegradation process. In comparison with petrodiesel, biodiesel shows better emission parameters. It improves the environmental performance of road transport and reduces greenhouse emissions (mainly of carbon dioxide) (2).
The use of biodiesel in a conventional diesel engine dramatically reduces the emissions of unburned hydrocarbons, carbon dioxide (CO2), carbon monoxide (CO), sulfates, polycyclic aromatic hydrocarbons, nitrated polycyclic aromatic hydrocarbons, ozone-forming hydrocarbons, and particulate matter (2). Oxygen content of biodiesel improves the combustion process and decreases its oxidation potential. Structural oxygen content of a fuel improves combustion efficiency due to the increase of the homogeneity of oxygen with the fuel during combustion. Because of this the combustion efficiency of biodiesel is higher than petrodiesel. Additionally,the use of biodiesel can extend the service life of diesel engines, as it is more lubricious than mineral diesel. Biodiesel has better lubricating properties than petrodiesel. The higher heating values (HHV) of biodiesel are relatively high. The HHVs of biodiesel (39–41 MJ/kg) are slightly lower than those of gasoline (46 MJ/kg), petrodiesel (43 MJ/kg) or petroleum (42 MJ/kg), but higher than those of coal (32–37 MJ/kg) (9).
Materials and Methods
Results and Discussion
Conclusion
Biodiesel is a better fuel than petro-diesel and meets most of the chemical/physical standards of petrodiesel. It has the potential to offer a number of benefits, such as economic, agricultural, environmental (due to its biodegradability, lower toxicity, renewability) and health (greenhouse gas savings, less harmful exhaust emissions). Biodiesel can be considered as the best option that has immense potential to fulfill the fuel requirements and secure a sustainable fuel supply in the future. In large-scale production plants, glycerin is usually recovered and purified, as it is a valuable substance that has numerous applications in the pharmaceutical, cosmetic and chemical industries. Future research could focus on optimizing the production process, investigating new feedstock sources, and exploring emerging markets for biodiesel.
References
🕮 1. Raqeeb MA, Bhargavi R, et al. (2015). Biodiesel production from waste cooking oil. J. Chem. Pharm. Res. 7 (12): 670-681.
🕮 2. Demirbas A. (2008). A Realistic Fuel Alternative for Diesel Engines. Energy Convers. Manag. 47 (15-16): 2271-2282. DOI: 10.1007/978-1-84628-995-8.
🕮 3. NanthaGopal K, Pal A, Sharma S, Samanchi C, Sathyanarayanan K, Elango T. (2014). Investigation of emissions and combustion characteristics of a CI engine fueled with waste cooking oil methyl ester and diesel blends. Alexandria Eng. J. 53: 281–287. https://doi.org/10.1016/j.aej.2014.02.003.
🕮 4. Carlini M, Castellucci S, Cocchi. (2014). A pilot-scale study of waste vegetable oil transesterification with alkaline and acidic catalysts. Energy Procedia. 45: 198-206. https://doi.org/10.1016/j.egypro.2014.01.022.
🕮 5. Budimanb, Kawentara WA, Arief. (2013). Synthesis of Biodiesel from Second-Used Cooking Oil.Energy Procedia. 32: 190 – 199. https://doi.org/10.1016/j.egypro.2013.05.025.
🕮 6. Rengel A, et al. (2008). Promising technologies for biodiesel production from algae growth systems. In The 8th European symposium of the international farming systems association, IFSA. Clermont-Ferrand, France.DOI: hal-00817352.
🕮 7. Mota CJA, Pinto BP, Lima AL. (2017). Glycerol. "Versatile Renewable Feedstock for the Chemical Industry. Cham: Springer.DOI: 10.1007/978-3-319-59375-3.
🕮 8. Ali MH, Mashud M, Rubel MR, et al. (2013). Biodiesel from Neem oil as an alternative fuel for Diesel engine. Procedia Eng. 56: 625 – 630. DOI:10.1016/j.proeng.2013.03.169.
🕮 9. Demirbas A. (2007). Importance of biodiesel as transportation fuel. Energy Policy. 35 (9): 4661-4670. https://doi.org/10.1016/j.enpol.2007.04.003.
🕮 10. Animasaun DA, Ameen MO, Belewu MA. (2021). Protocol for Biodiesel Production by Base-Catalyzed Transesterification Method. In: Biofuels and Biodiesel. New York: Springer US. p. 103-113.DOI: 10.1007/978-1-0716-1323-8_7
🕮 11. Song C. (2000). Chemistry of diesel fuels. CRC Press.https://doi.org/10.1201/9781003075455.
🕮 12. Demirbas A. (2006). Biodiesel production via non-catalytic SCF method and biodiesel fuel characteristics. Energy Convers. Manag. 47 (15-16): 2271-2282.DOI:10.1016/j.enconman.2005.11.019.
🕮 13. Ogbu IM, Ajiwe VIE. (2016). Fuel properties and their correlations with fatty acids structures of methyl-and butyl-esters of Afzeliaafricana, Cucurbitapepo and Huracrepitans seed oils. Waste Biomass Valor. 7: 373-381.DOI:10.1007/s12649-015-9446-4.
🕮 14. Atabani AE, Silitonga AS, Ong HC, et al. (2013). Non-edible vegetable oils: a critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew. Sustain. Energy Rev. 18: 211-245.DOI: 10.1016/j.rser.2012.10.013.
🕮 15. Dias JM, Alvim-Ferraz MCM, Almeida MF. (2008). Comparison of the performance of different homogeneous alkali catalysts during transesterification of waste and virgin oils and evaluation of biodiesel quality. Fuel. 87 (17-18).DOI:10.1016/j.fuel.2008.06.014.
🕮 16. Budiman A, Kusumaningtyas RD, Pradana YS, et al. (2018). Biodiesel:Bahan Baku, Proses, danTeknologi: Bahan Baku, Proses, danTeknologi. Ugm Press.
🕮 17. Banerjee N, Ramakrishnan R, Jash T. (2014). Biodiesel production from used vegetable oil collected from shops selling fritters in Kolkata. Energy Procedia. 54: 161-165.DOI:10.1016/j.egypro.2014.07.259.
🕮 18. Islam MN, Beg MRA. (2004). The fuel properties of pyrolysis liquid derived from urban solid wastes in Bangladesh. Bioresour. Technol. 92 (2): 181-186.DOI: 10.1016/j.biortech.2003.08.009.
🕮 19. Yesilyurt MK, Cesur C. (2020). Biodiesel synthesis from Styraxofficinalis L. seed oil as a novel and potential non-edible feedstock: A parametric optimization study through the Taguchi technique. Fuel. 265 : 117025.DOI:10.1016/j.fuel.2020.117025.
🕮 20. Yesilyurt MK, Cesur C, Aslan V, et al. (2020). The production of biodiesel from safflower (Carthamustinctorius L.) oil as a potential feedstock and its usage in compression ignition engine: A comprehensive review. Renew. Sustain. Energy Rev. 119: 109574.https://doi.org/10.1016/j.rser.2019.109574.
🕮 21. Rahadianti ES, Yerizam, Martha. (2018). Biodiesel production from waste cooking oil. IJFAC (Indonesian J. Fundam. Appl. Chem.). 3 (3): 77-82. DOI: 10.24845/ijfac.v3.i3.77.