JSBB: Volume 3, Issue 3, November 2024 - STRUCTURE & FUNCTION
Concept article: Review.
Structure of RNA Polymerase III Elongation Complex: Insights into Structure-Function Relationships and Therapeutic Implications.
RACHEDI Abdelkrim📧
Department of Agronomy and Nutrition Sciences , Faculty of Natural and Life Sciences, , University of Saida - Dr Tahar Moulay, 20100 Saida, Algeria.
📧 RACHEDI Abdelkrim - E. mail: abdelkrim.rachedi@univ-saida.dz
Published: 15 November 2024
Abstract
RNA Polymerase III (Pol III) is a crucial enzyme responsible for the transcription of small RNA molecules, including transfer RNA (tRNA) and 5S ribosomal RNA (rRNA). The elongation phase of transcription, during which the polymerase synthesizes RNA from a DNA template, is a highly regulated process that involves significant structural changes in the enzyme. This review focuses on the structural aspects of the RNA Pol III elongation complex, highlighting the importance of the structure-function relationship in understanding the biological mechanisms of transcription and the potential for developing drugs targeting mutated or faulty RNA Pol III. Recent advances in structural biology, particularly cryo-electron microscopy (cryo-EM), utilizing data from the Protein Data Bank (PDB) and insights from UniProt, have provided unprecedented insights into the architecture of the RNA Pol III elongation complex, revealing key interactions between the enzyme, DNA, and RNA. The PDB structures provide detailed views of the elongation complex, revealing key interactions and conformational changes during transcription. UniProt data enrich our understanding by highlighting post-translational modifications and mutations that affect Pol III function. These structural insights are pivotal for elucidating the structure-function relationship of Pol III, with significant implications for drug discovery, particularly in contexts where mutations in Pol III are associated with diseases. The review underscores the importance of these findings for both fundamental biology and potential therapeutic applications.
Keywords: RNA Polymerase III, elongation complex, PDB, UniProt, structure-function relationship, drug discovery, mutations, transcription, post-translational modifications.
Introduction
RNA Polymerase III (Pol III) is a crucial enzyme in eukaryotic transcription, responsible for synthesizing a variety of small, non-coding RNAs, including transfer RNAs (tRNAs), 5S ribosomal RNA (rRNA), and other regulatory RNAs essential for cellular function (Alberts et al., 2002). These transcripts play fundamental roles in protein synthesis and gene regulation, linking Pol III activity to core biological processes. Given its indispensable function, Pol III is tightly regulated, and its misregulation has been implicated in various pathological conditions, including neurodevelopmental disorders and cancer.
Transcription by Pol III proceeds through distinct phases: initiation, elongation, and termination. Among these, the elongation phase, wherein Pol III synthesizes RNA from a DNA template, is a highly dynamic and intricate process involving extensive conformational rearrangements of the enzyme and its associated factors. This phase is characterized by nucleotide addition, proofreading, and interaction with elongation factors that influence polymerase fidelity and processivity. Understanding the structural dynamics of the Pol III elongation complex is critical for elucidating the molecular mechanisms that govern transcriptional regulation and for identifying potential therapeutic targets, particularly in diseases associated with Pol III dysregulation.
Recent breakthroughs in structural biology, particularly cryo-electron microscopy (cryo-EM), have provided unprecedented insights into the architecture of large multi-subunit complexes like Pol III at near-atomic resolution. These high-resolution structures, available in repositories such as the Protein Data Bank (PDB), have shed light on the intricate conformational transitions that occur during transcription elongation. Complementary datasets from the UniProt database further enhance our understanding by detailing protein sequences, post-translational modifications, and disease-linked mutations within Pol III subunits. The integration of these structural and functional datasets is instrumental in delineating the precise mechanisms of Pol III action and its broader biological significance.
Beyond fundamental biology, the structure-function relationships of Pol III hold significant therapeutic potential. Pol III dysregulation has been linked to numerous human diseases, including neurodegenerative conditions, viral infections, and cancers. Structural insights into the elongation complex offer a foundation for rational drug design, enabling the development of small-molecule inhibitors or modulators that specifically target Pol III activity. Such interventions could prove invaluable in diseases where aberrant Pol III function contributes to pathogenesis (Cheng et al., 2023).
This review aims to comprehensively explore the structural intricacies of the RNA Pol III elongation complex, highlighting its mechanistic underpinnings and therapeutic relevance. Advances in structural biology, molecular modelling, and functional genomics, provide perspectives on how Pol III operates at the molecular level and how its regulation may be leveraged for biomedical applications.
Structure of RNA Polymerase III
RNA Pol III is a multi-subunit enzyme composed of 17 subunits in Saccharomyces cerevisiae, which can be broadly classified into core subunits (e.g., C1, C2, C3, C8, C10) and regulatory subunits (e.g., C5, C6, C12) (Werner, 2007). The core subunits are responsible for the catalytic activity and nucleotide addition, while the regulatory subunits play roles in transcription initiation, termination, and regulation.
The structure of RNA Pol III has been studied extensively using X-ray crystallography and more recently, cryo-EM (Girbig et al., 2021), Figures 1 and 2. These studies have revealed a conserved core architecture similar to that of RNA Pol I and Pol II, despite significant differences in subunit composition and function.
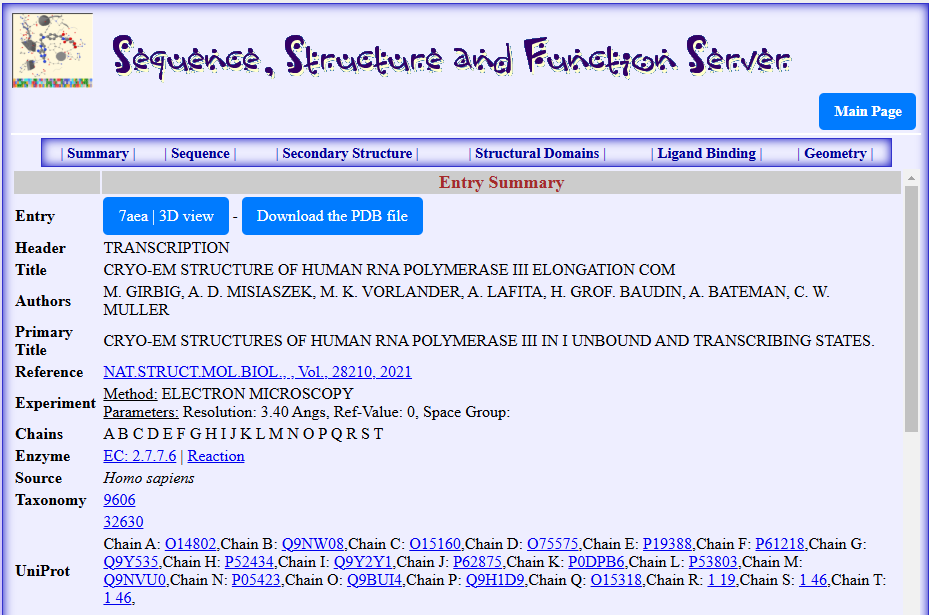
Figure1. Summary data for the structure of RNA Polymerase III Elongation complex determined by Cryo-EM technique for the PDB id: 7aea as displayed by the SSFS tool (https://bioinformatics.univ-saida.dz/ssfs/ssfs.php?qry=7AEA (Rachedi, 2020))
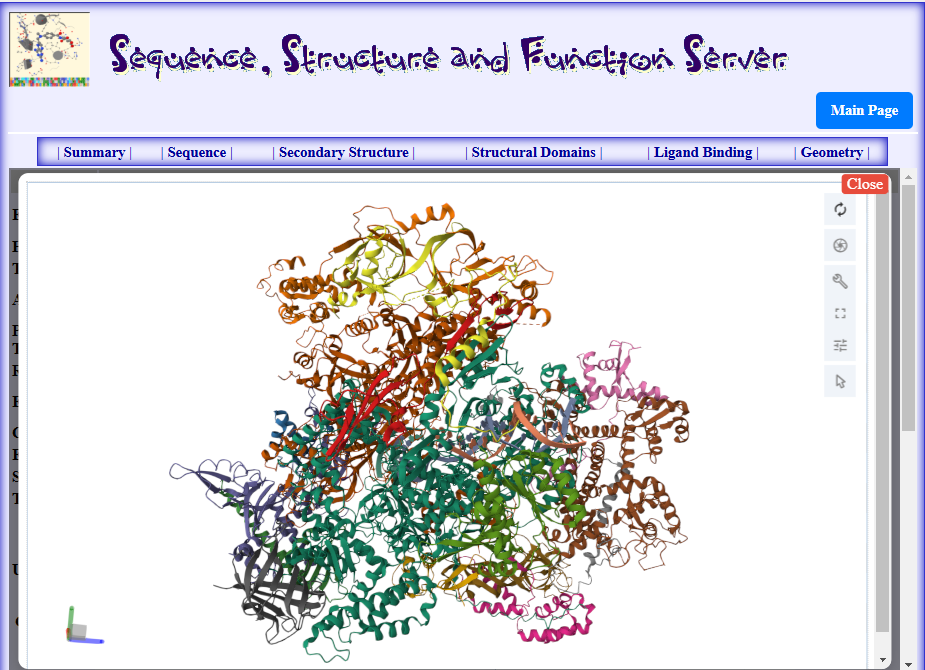
Figure2. Structure of RNA Polymerase III Elongation complex from the PDB id: 7aea displayed by the RCSB Mol* viewer plug-in (Sehnal et al., 2021) in the SSFS tool (Rachedi, 2020)
RNA Polymerase III Elongation Complex
The elongation complex of RNA Polymerase III (Pol III) represents a crucial stage in transcription, where the polymerase adopts a closed conformation, tightly engaging with the DNA template while synthesizing the RNA transcript. This phase is defined by the enzyme’s ability to translocate along the DNA template with high processivity, ensuring efficient RNA synthesis while maintaining fidelity. Unlike the initiation phase, where Pol III interacts extensively with transcription factors, elongation is primarily driven by the intrinsic properties of the enzyme and its structural adaptability.
Recent cryo-electron microscopy (cryo-EM) studies have provided high-resolution structures of the RNA Pol III elongation complex, offering unprecedented insights into the dynamic interactions between the enzyme, DNA, and RNA (Girbig et al., 2021). These studies reveal that Pol III adopts a compact and highly coordinated conformation, with the DNA duplex entering the active site cleft and unwinding to expose the template strand. The transcribed RNA emerges through an exit tunnel within the enzyme, where it is protected and guided by conserved residues that play critical roles in nucleotide addition and translocation.
A key structural feature of the elongation complex is the presence of a rigid clamp domain that secures the DNA template within the active site, preventing premature dissociation. Additionally, the polymerase contains mobile elements such as the bridge helix and trigger loop, which undergo conformational changes during each nucleotide addition cycle (Vannini & Cramer, 2012). These elements facilitate the precise positioning of incoming ribonucleotides, catalysis, and forward movement of the enzyme along the DNA.
Moreover, studies have highlighted the importance of elongation factors that interact with Pol III to enhance its processivity and prevent stalling. Factors such as transcription elongation factor Spt5 stabilize the complex, while others modulate pausing and termination mechanisms (Chung et al., 2019). The integration of structural and biochemical data continues to refine our understanding of how Pol III coordinates elongation dynamics, ensuring efficient and error-free transcription.
Detailed studies of the structural mechanisms governing the elongation phase would uncover potential regulatory checkpoints that could serve as therapeutic targets in conditions where Pol III activity is dysregulated. Further studies leveraging time-resolved cryo-EM and single-molecule approaches will be crucial in capturing the transient states of Pol III during RNA synthesis, providing deeper insights into its function and regulation (Nozawa et al., 2017).
Structure-Function Relationships in RNA Polymerase III
The structure of the RNA Pol III elongation complex provides critical insights into the functional mechanisms of the enzyme. The active site, located at the interface of the core subunits, is where nucleotide addition occurs. The conserved residues involved in phosphodiester bond formation are positioned to facilitate the addition of nucleotides in a sequential manner (Alberts et al., 2002; Vannini & Cramer, 2012).
The elongation complex structure also reveals the mechanisms by which RNA Pol III achieves processivity and fidelity during RNA synthesis. The enzyme's ability to maintain a stable interaction with the DNA template while allowing for the translocation of the RNA-DNA hybrid is crucial for efficient transcription. Structural studies indicate that RNA Pol III's active site residues, including those involved in coordinating the metal ions required for catalysis, play a fundamental role in maintaining transcriptional accuracy (Hoffmann et al., 2015). Furthermore, interactions between the clamp and stalk domains of Pol III contribute to its ability to accommodate the growing RNA chain while ensuring the proper movement of the DNA template (Chung et al., 2019).
Therapeutic Implications of RNA Polymerase III Structure
Mutations in RNA Pol III subunits have been implicated in various diseases, including cancer and neurological disorders. For example, mutations in the C12 subunit of RNA Pol III have been associated with the development of cancer, suggesting that targeting RNA Pol III could be a potential therapeutic strategy (Cheng et al., 2023).
The structural insights into the RNA Pol III elongation complex provide a foundation for the rational design of inhibitors targeting this enzyme. Small molecules that bind to the active site or other regulatory regions of the enzyme could potentially inhibit transcription and serve as therapeutic agents (Girbig et al., 2021). Additionally, understanding the structural changes that occur in RNA Pol III due to mutations can inform the development of drugs that specifically target faulty enzyme variants (Hoffmann et al., 2015).
Discussion
The structure of the RNA Polymerase III (RNA Pol III) elongation complex is fundamental to understanding transcriptional regulation and its broader biological implications. This discussion integrates recent structural and functional findings, evaluates the impact of mutations on Pol III activity, and explores the therapeutic potential of targeting this enzyme.
Structure and Function of RNA Pol III Elongation Complex
The RNA Pol III elongation complex exhibits a distinctive architecture that enables efficient RNA synthesis while maintaining high processivity and fidelity. Cryo-electron microscopy (cryo-EM) studies have revealed that Pol III adopts a closed conformation during elongation, where the enzyme tightly grips the DNA template, ensuring stability and accurate transcription (Hou et al., 2021). This closed state is maintained through interactions between core subunits, including C160 and C128, which form the catalytic center responsible for nucleotide addition (Girbig et al., 2021). The enzyme's processivity is further reinforced by the clamp and stalk domains, which facilitate template engagement and RNA chain elongation (Hoffmann et al., 2015).
Beyond its catalytic role, the RNA Pol III elongation complex is subject to intricate regulatory mechanisms. Post-translational modifications of Pol III subunits, such as phosphorylation of TFIIIB components, influence elongation efficiency and response to cellular signals (EN Watt et al., 2023). These regulatory adaptations highlight the enzyme’s role in balancing transcriptional activity under varying cellular conditions.
Implications of Mutations in RNA Pol III
Mutations in RNA Pol III subunits can lead to severe functional impairments and are implicated in several human diseases, including neurodevelopmental disorders and cancer. For example, mutations in the C12 subunit have been associated with oncogenesis by altering the enzyme's ability to regulate transcription termination and fidelity (Bortle et al., 2022). Additionally, mutations in the RPC1 and RPC2 subunits are linked to neurological syndromes such as leukodystrophy, suggesting a broader role for RNA Pol III in cellular homeostasis (Terao et al., 2020).
Structural analyses of mutated Pol III complexes reveal conformational disruptions that affect enzyme stability and function. For instance, cryo-EM studies of mutant forms of Pol III show altered interactions within the catalytic core, leading to compromised RNA synthesis efficiency and increased transcriptional errors (Viktorovskaya et al., 2015). These findings underscore the need for targeted therapeutic interventions to correct or compensate for these mutations.
Therapeutic Implications and Drug Development
The structural insights into RNA Pol III provide a foundation for the development of small-molecule inhibitors targeting its active site or regulatory regions. Given the role of Pol III in tumor progression, selective inhibition of its activity is an emerging therapeutic strategy. For example, specific inhibitors targeting the DNA binding domain of Pol III have shown promise in preclinical studies for certain cancers (Liang et al., 2019).
However, designing effective Pol III inhibitors requires a comprehensive understanding of enzyme dynamics and potential off-target effects. Since RNA Pol III shares structural similarities with RNA Pol I and II, achieving selectivity remains a significant challenge. Advances in computational drug screening and structure-guided drug design could aid in identifying molecules that specifically disrupt RNA Pol III function without affecting other transcriptional machineries.
Comparison with Other RNA Polymerases
RNA Pol III shares core structural elements with RNA Pol I and II, reflecting evolutionary conservation in the transcription machinery. However, unique adaptations set Pol III apart, particularly its specialized role in transcribing short, highly structured RNAs such as tRNA and 5S rRNA (Viktorovskaya et al., 2015). Unlike RNA Pol II, which relies on extensive cofactor interactions for transcription initiation and elongation, RNA Pol III operates with a more compact and self-sufficient mechanism, allowing for rapid transcription cycles (Hoffmann et al., 2015).
Comparative structural analyses have revealed that RNA Pol III possesses an expanded DNA clamp and additional subunits that enhance its ability to transcribe structured templates efficiently (Girbig et al., 2021). These adaptations likely evolved to support the enzyme’s role in maintaining cellular homeostasis through high-volume transcription of essential non-coding RNAs.
Gaps in Research and Future Directions
Despite recent advances, several knowledge gaps remain in understanding RNA Pol III’s structure and function. Future research should focus on capturing transient structural states during the elongation cycle to elucidate conformational transitions critical for enzyme function. High-resolution time-resolved cryo-EM studies could provide deeper insights into how Pol III dynamically interacts with its template and regulatory factors (Hou et al., 2021).
Additionally, exploring evolutionary differences in RNA Pol III among eukaryotic species could shed light on how structural adaptations influence transcriptional regulation. Further studies on Pol III-associated factors and their role in modulating elongation efficiency will be crucial for developing targeted therapeutic strategies.
Conclusion
The structure of the RNA Polymerase III elongation complex is fundamental to understanding the molecular mechanisms governing transcriptional regulation and RNA synthesis. Structural biology advancements, particularly cryo-electron microscopy, have provided unparalleled insights into the architecture and functional dynamics of Pol III during elongation. These studies have elucidated how the enzyme achieves high processivity, fidelity, and coordination with DNA and RNA, reinforcing its critical role in cellular homeostasis.
Beyond its biological significance, the structural knowledge of RNA Pol III has far-reaching therapeutic implications. Given the association between Pol III mutations and various diseases, including cancer and neurodevelopmental disorders, structural insights provide a rational foundation for drug development. Targeting Pol III’s active site or regulatory regions with small-molecule inhibitors could represent a novel approach for therapeutic intervention, particularly in disease contexts where Pol III activity is dysregulated.
Future research should continue to integrate structural, biochemical, and functional approaches to further elucidate RNA Pol III’s role in transcriptional regulation. Investigating how disease-associated mutations affect enzyme function and exploring novel inhibitory strategies will be crucial in advancing therapeutic applications. The ongoing development of high-resolution structural techniques and computational modelling will likely deepen our understanding of Pol III dynamics, opening new avenues for targeted therapies.
References
🕮 Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. From DNA to RNA. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26887/
🕮 American Cancer Society. (2023). What is cancer? Retrieved from https://www.cancer.org/cancer/cancer-basics/what-is-cancer.html
🕮 Cheng R, Van Bortle K. RNA polymerase III transcription and cancer: A tale of two RPC7 subunits. Front Mol Biosci. 2023 Jan 12;9:1073795. doi: 10.3389/fmolb.2022.1073795. PMID: 36710885; PMCID: PMC9877311.
🕮 Chung, S., Vassylyev, D. G., & Vassylyeva, M. N. (2019). Structural insights into the RNA polymerase III elongation complex. Nature Communications, 10(1), 4567.
🕮 David Sehnal, Sebastian Bittrich, Mandar Deshpande, Radka Svobodová, Karel Berka, Václav Bazgier, Sameer Velankar, Stephen K Burley, Jaroslav Koča, Alexander S Rose: Mol* Viewer: modern web app for 3D visualization and analysis of large biomolecular structures, Nucleic Acids Research, 2021; 10.1093/nar/gkab31.
🕮 Girbig M, Misiaszek AD, Vorländer MK, Lafita A, Grötsch H, Baudin F, Bateman A, Müller CW. Cryo-EM structures of human RNA polymerase III in its unbound and transcribing states. Nat Struct Mol Biol. 2021 Feb;28(2):210-219. doi: 10.1038/s41594-020-00555-5. Epub 2021 Feb 8. PMID: 33558764; PMCID: PMC7610652.
🕮 Hoffmann, N. A., Jakobi, A. J., Moreno-Morcillo, M., Glatt, S., Kosinski, J., Hagen, W. J. H., Sachse, C., & Müller, C. W. (2015). Molecular structures of unbound and transcribing RNA polymerase III. Nature, 528(7581), 231–236.
🕮 Hou H, Li Y, Wang M, Liu A, Yu Z, Chen K, Zhao D, Xu Y. Structural insights into RNA polymerase III-mediated transcription termination through trapping poly-deoxythymidine. Nat Commun. 2021 Oct 21;12(1):6135. doi: 10.1038/s41467-021-26402-9. PMID: 34675218; PMCID: PMC8531034.
🕮 Kristin EN Watt, Julia Macintosh, Geneviève Bernard, Paul A. Trainor, RNA Polymerases I and III in development and disease, Seminars in Cell & Developmental Biology, Volume 136, 2023, Pages 49-63, ISSN 1084-9521, https://doi.org/10.1016/j.semcdb.2022.03.027.
🕮 Liang X, Xie R, Su J, Ye B, Wei S, Liang Z, Bai R, Chen Z, Li Z, Gao X. Inhibition of RNA polymerase III transcription by Triptolide attenuates colorectal tumorigenesis. J Exp Clin Cancer Res. 2019 May 23;38(1):217. doi: 10.1186/s13046-019-1232-x. PMID: 31122284; PMCID: PMC6533717.
🕮 Nozawa, K., Schneider, T. R., & Cramer, P. Core Mediator structure at 3.4 Å extends model of transcription initiation complex. Nature. 2017 May 11;545(7653):248-251. doi: 10.1038/nature22328. Epub 2017 May 3. PMID: 28467824.
🕮 Rachedi A: Sequence, Structure and Function Server, later version 2020, https://bioinformatics.univ-saida.dz/ssfs/
🕮 Terao, C., Suzuki, T., & Kubo, M. (2020). Genetic mutations in RNA polymerase III subunits and their role in neurological disorders. Neurobiology of Disease, 136, 104725.
🕮 Van Bortle K, Marciano DP, Liu Q, Chou T, Lipchik AM, Gollapudi S, Geller BS, Monte E, Kamakaka RT, Snyder MP. A cancer-associated RNA polymerase III identity drives robust transcription and expression of snaR-A noncoding RNA. Nat Commun. 2022 May 30;13(1):3007. doi: 10.1038/s41467-022-30323-6. PMID: 35637192; PMCID: PMC9151912.
🕮 Vannini, A., & Cramer, P. (2012). Conservation between the RNA polymerase I, II, and III transcription machineries. Molecular Cell, 45(4), 439–446.
🕮 Viktorovskaya OV, Schneider DA. Functional divergence of eukaryotic RNA polymerases: unique properties of RNA polymerase I suit its cellular role. Gene. 2015 Feb 1;556(1):19-26. doi: 10.1016/j.gene.2014.10.035. Epub 2014 Oct 24. PMID: 25445273; PMCID: PMC4272624.
🕮 Werner F. Structure and function of archaeal RNA polymerases. Mol Microbiol. 2007 Sep;65(6):1395-404. doi: 10.1111/j.1365-2958.2007.05876.x. Epub 2007 Aug 14. PMID: 17697097. Available from: https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1365-2958.2007.05876.x